| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 499-506
Effectiveness of Antibiotic Regimens in Reducing White Blood Cell Count Within Three to Five Days in Febrile Leukocytosis Treated With Ambulatory Therapy
Woraphat Tumporna , Thanin Lokeskraweea, e
, Natthaphon Pruksathorna
, Suppachai Lawanaskolb
, Jayanton Patumanondc, Suwapim Chanlaord, Wanwisa Bumrungpagdeed, Chawalit Lakdeed
aDepartment of Emergency Medicine, Lampang Hospital, Lampang 52000, Thailand
bChaiprakarn Hospital, Chiang Mai 50320, Thailand
cClinical Epidemiology and Clinical Statistics Unit, Faculty of Medicine, Naresuan University, Phitsanulok 65000, Thailand
dDepartment of Radiology, Buddhachinaraj Phitsanulok Hospital, Phitsanulok 65000, Thailand
eCorresponding Author: Thanin Lokeskrawee, Department of Emergency Medicine, Lampang Hospital, Lampang 52000, Thailand
Manuscript submitted July 30, 2025, accepted October 8, 2025, published online October 15, 2025
Short title: IV Antibiotics for Fever, Leukocytosis
doi: https://doi.org/10.14740/jocmr6339
| Abstract | ▴Top |
Background: In the absence of sepsis, patients with fever and leukocytosis in the emergency department (ED) are often treated with ambulatory parenteral antibiotics at the physician’s discretion. Identifying effective regimens for reducing white blood cell (WBC) count and improving clinical outcomes may support standardized ED care.
Methods: This retrospective cohort included adult ED patients with fever and leukocytosis, but without sepsis, in whom basic investigation revealed no clear source of infection. Patients received one of three regimens: (A) single-day intravenous (IV) ceftriaxone followed by oral cefixime, (B) 3-day IV ceftriaxone followed by oral cefixime, or (C) 3-day IV ceftriaxone plus oral doxycycline from day 1. Demographics, baseline data, and laboratory results were collected. Follow-up assessments included WBC count and clinical improvement. Treatment probabilities were estimated with multinomial logistic regression, and stabilized inverse probability of treatment weighting (IPTW) were applied in weighted quantile regression.
Results: Among 250 patients, most were female (63.2%) with a mean age of 49 years. After adjustment, regimen C showed a median WBC reduction of -6.9 × 103 cells/mm3 (95% confidence interval (CI): -7.5 to -6.3), compared with -6.8 × 103 (95% CI: -7.2 to -6.5) for regimen B and -6.6 × 103 (95% CI: -7.0 to -6.2) for regimen A. Differences were not significant (Wald test, P = 0.484), but graphical analysis suggested the steepest decline with regimen C.
Conclusion: Though regimen C showed the steepest WBC decline and fewer failures, the study was markedly underpowered (< 10%). Larger multicenter studies are required to confirm these findings.
Keywords: Fever; Leukocytosis; Anti-bacterial agents; Ambulatory care; Treatment outcome; Leukocyte count
| Introduction | ▴Top |
Fever with leukocytosis is a common presentation in the emergency department (ED), with a prevalence of approximately 10.1% based on retrospective data from Lampang Hospital, whereas the prevalence of sepsis in the ED, when calculated using all chief complaints as the denominator, has been reported at 0.89% [1]. All patients diagnosed with sepsis were admitted. In those without sepsis, a focused initial evaluation, including vital signs, history, and physical examination, was crucial to identify the infectious source. Timely, targeted antibiotic therapy based on the suspected site remains key to improving clinical outcomes and minimizing antimicrobial resistance.
For the ED setting, the concept was adapted from the original 1974 work by Rucker and Harrison, which introduced outpatient parenteral antimicrobial therapy (OPAT) to shorten hospital stays. They first administered intravenous (IV) antibiotics at home for children with cystic fibrosis [2], laying the groundwork for modern OPAT programs. Currently, OPAT is commonly used as a continuation of care post-discharge from inpatient hospitalization, for conditions such as cellulitis, urinary tract infections (UTIs), and pneumonia [3, 4].
There are two main types of OPAT: 1) home-based OPAT, which requires patient or caregiver education for self-administration. This approach is often impractical in developing countries due to safety concerns; 2) hospital-based (ambulatory) OPAT, where patients return to the hospital daily for IV antibiotics. This safer, more feasible model is commonly used in Thailand. In most ED cases, treatment duration typically does not exceed 3 days.
At the ED of Lampang Hospital, febrile patients presenting with leukocytosis who undergo initial assessment, comprehensive physical examination, and basic laboratory investigations, but without an identifiable source of infection, are often managed with OPAT rather than hospitalization. This approach is more practical, especially since inpatient investigations also frequently fail to reveal a definitive pathogen. Therefore, this study aims to identify the most effective OPAT regimen for symptom improvement and reduction of white blood cell (WBC) count.
| Materials and Methods | ▴Top |
Study design
This therapeutic research employed a retrospective observational cohort design, conducted at Lampang Hospital, a tertiary care center in Northern Thailand, from January 2012 to December 2024. The hospital manages approximately 500,000 outpatient visits and about 90,000 emergency visits annually.
Febrile patients with leukocytosis deemed suitable for OPAT were assigned treatment regimens based on physician discretion. A follow-up protocol was established for reassessment, including repeat complete blood count and urine analysis (UA), within 3 to 5 days.
Data collected included sex, age, presenting symptoms, comorbidities, vital signs, focused investigation on the presenting symptoms, initial WBC, and initial polymorphonuclear neutrophil (PMN) percentage. At follow-up, clinical improvement, appetite, vital signs, UA, WBC, and PMN percentage were also recorded.
Participant and data collection
Participants
Adult patients (≥ 18 years) with fever > 37.8 °C measured by infrared thermometer [5] and leukocytosis (WBC > 11,000 cells/mm3) [6, 7], who had stable vital signs, no suspicion of sepsis as indicated by a National Early Warning Score (NEWS) < 5 [8, 9], blood lactate < 2 mmol/L [10], and were considered suitable for OPAT were included. Importantly, these patients underwent basic investigation in the ED, and when no clear source of infection was identified, they were included in the study domain. Exclusion criteria included development of sepsis during ED or emergency department observation unit (EDOU) stay, inability to complete follow-up evaluation, absence of follow-up blood tests, immunocompromised status, or known allergy to ceftriaxone or doxycycline.
Determinant (Xs): Lampang Hospital’s ED OPAT protocol
Patients suitable for OPAT were identified only if they passed all five components of the rapid triage “Sick or Not Sick” assessment [11]: 1) visual cues, 2) vital signs, 3) risk factors, 4) good appetite, and 5) ability to ambulate without difficulty, ensuring that only patients in genuinely good condition were prescribed OPAT. Three main regimens were available for ambulatory OPAT (A) single-day IV ceftriaxone followed by oral cefixime; (B) 3-day course of IV ceftriaxone followed by oral cefixime; or (C) 3-day course of IV ceftriaxone plus oral doxycycline initiated on day 1. Regimen selection was based on physician preference, patient age, initial WBC count, PMN percentage, and individual risk factors. The study was purely observational, with no intervention by investigator.
Endpoints
The primary endpoint was WBC reduction, calculated as follow-up WBC minus pre-treatment WBC. A negative value indicated a reduction in WBC, whereas a positive value reflected an increase. WBC reduction per day was not calculated, as the primary clinical interest at follow-up was the absolute change from baseline rather than the rate of decline.
At follow-up, patients were asked about their clinical status (“better”, “same”, or “worse”). The secondary endpoints were: 1) clinical improvement, indicated by resolution of fever with a self-reported “better” status and a post-treatment WBC < 11 × 103 cells/mm3; and 2) treatment failure, which included (a) post-treatment WBC > 11 × 103 cells/mm3 with a self-reported “same” status, requiring extension or switch of IV ambulatory antibiotic therapy, or (b) worsening clinical status with post-treatment WBC still > 11 × 103 cells/mm3, requiring hospital admission for further pathogen workup.
Confounders
Confounders included sex, age, symptoms, comorbidities, initial WBC, and initial PMN percentage.
Study size estimation
Based on a pilot study of 25 patients that assessed the mean difference in WBC reduction between regimen A and regimen C (-1,600 ± 1,414 vs. -3,560 ± 5,546), a two-sided test with an alpha of 0.05 and power of 80% indicated a required sample size of 69 patients per group. Using the regimen ratio A:B:C = 1:1.8:1, the total calculated sample size was 259 patients (69 + 121 + 69).
Statistical analysis
Baseline characteristics were compared among the three treatment regimens. Categorical variables were analyzed using the exact probability test, while continuous variables were assessed using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test, depending on the data distribution. Given the observational nature of the study and the potential for selection bias related to treatment indications and contraindications, treatment group assignment (regimen A, B, or C) was modeled using multinomial logistic regression [12, 13] to address confounding by indication, with sex, age, symptoms, comorbidities, initial WBC, and initial PMN percentage included as covariates. Predicted probabilities for each regimen were then generated from the model. Inverse probability of treatment weighting (IPTW) [14] was applied by calculating the stabilized weights as the inverse of the predicted probability of receiving the treatment actually administered. After weighting, quantile regression analysis was performed to compare WBC reduction at 3-day follow-up across the treatment groups as the primary outcome. A global comparison was first performed using the Wald test, and pairwise comparisons with Bonferroni adjustment were also reported. Secondary outcomes were compared using the exact probability test.
The study protocol was registered in the Thai Clinical Trials Registry (TCTR) (TCTR20250726004) and approved by the Institutional Review Board of Lampang Hospital (CERT No. 120/67). Informed consent was waived because of the observational design of the study.
| Results | ▴Top |
Between January 2012 and December 2024, 432 patients were considered suitable for OPAT. After applying the exclusion criteria, 250 patients remained eligible, comprising 64, 128, and 58 patients in regimens A, B, and C, respectively (Fig. 1).
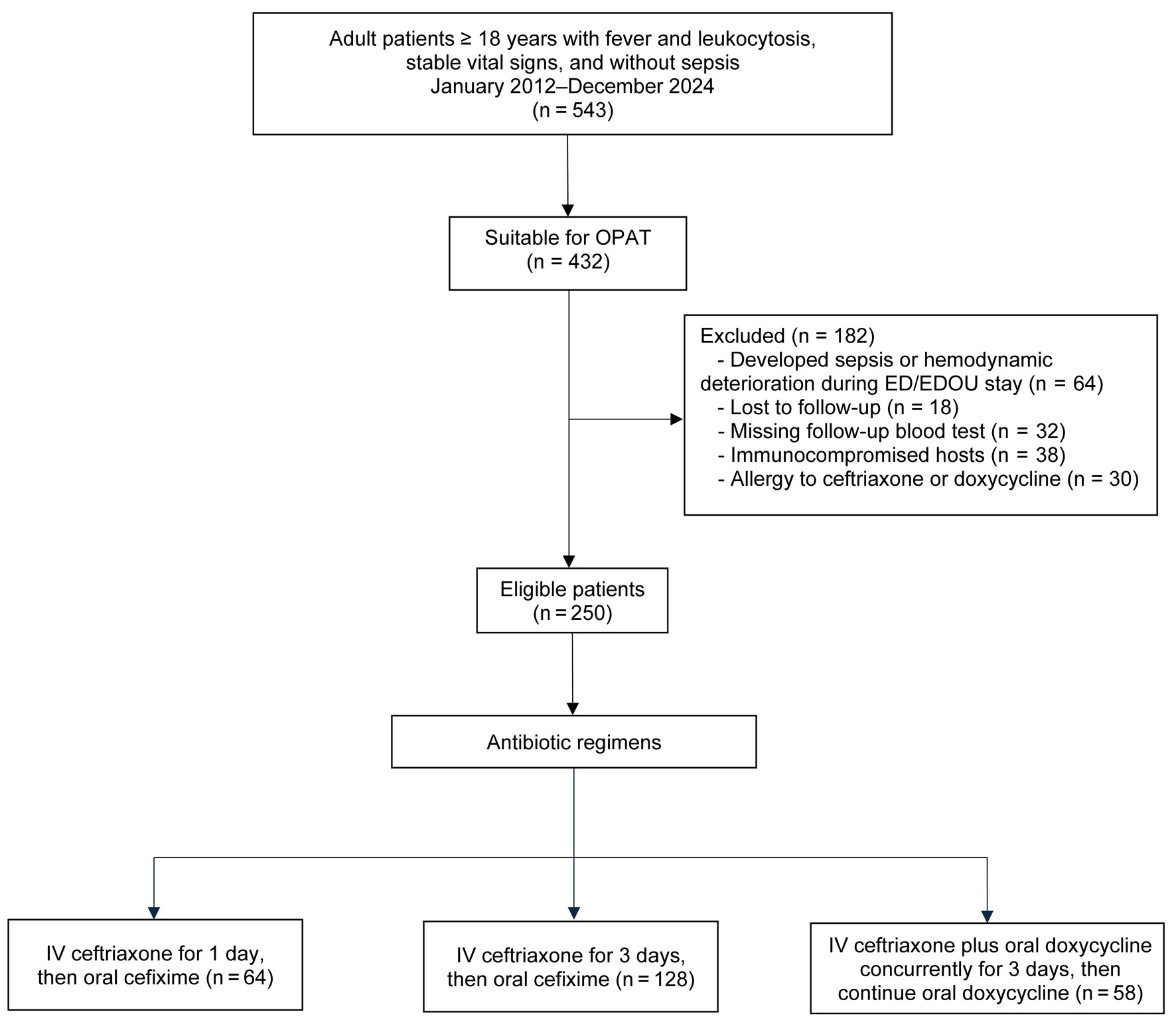 Click for large image | Figure 1. Study flow diagram. |
Female patients were most prevalent in regimen A, followed by regimen B and regimen C. Patients in regimen A were the youngest, whereas those in regimen B were the oldest. Across all three regimens, non-specific symptoms were the most frequent presentation. No significant differences were observed in other baseline characteristics between the regimens (Table 1).
 Click to view | Table 1. Baseline Characteristics |
Regimen B showed the highest crude pre-treatment WBC, with similar values between regimens A and C, though not statistically significant. Post-treatment WBC levels were comparable across all regimens, with no significant differences observed (Table 2).
 Click to view | Table 2. Crude Pre- and Post-Treatment White Blood Cell Counts Across Three Antibiotic Regimens |
After applying confounder-adjusted IPTW and quantile regression, regimen C showed the greatest median WBC reduction at -6.9 × 103 cells/mm3 (95% confidence interval (CI): -7.5 to -6.3), followed by regimen B at -6.8 × 103 cells/mm3 (95% CI: -7.2 to -6.5) and regimen A at -6.6 × 103 cells/mm3 (95% CI: -7.0 to -6.2). However, the overall Wald test indicated no significant differences among the regimens (P = 0.484) (Fig. 2; Tables 3 and 4).
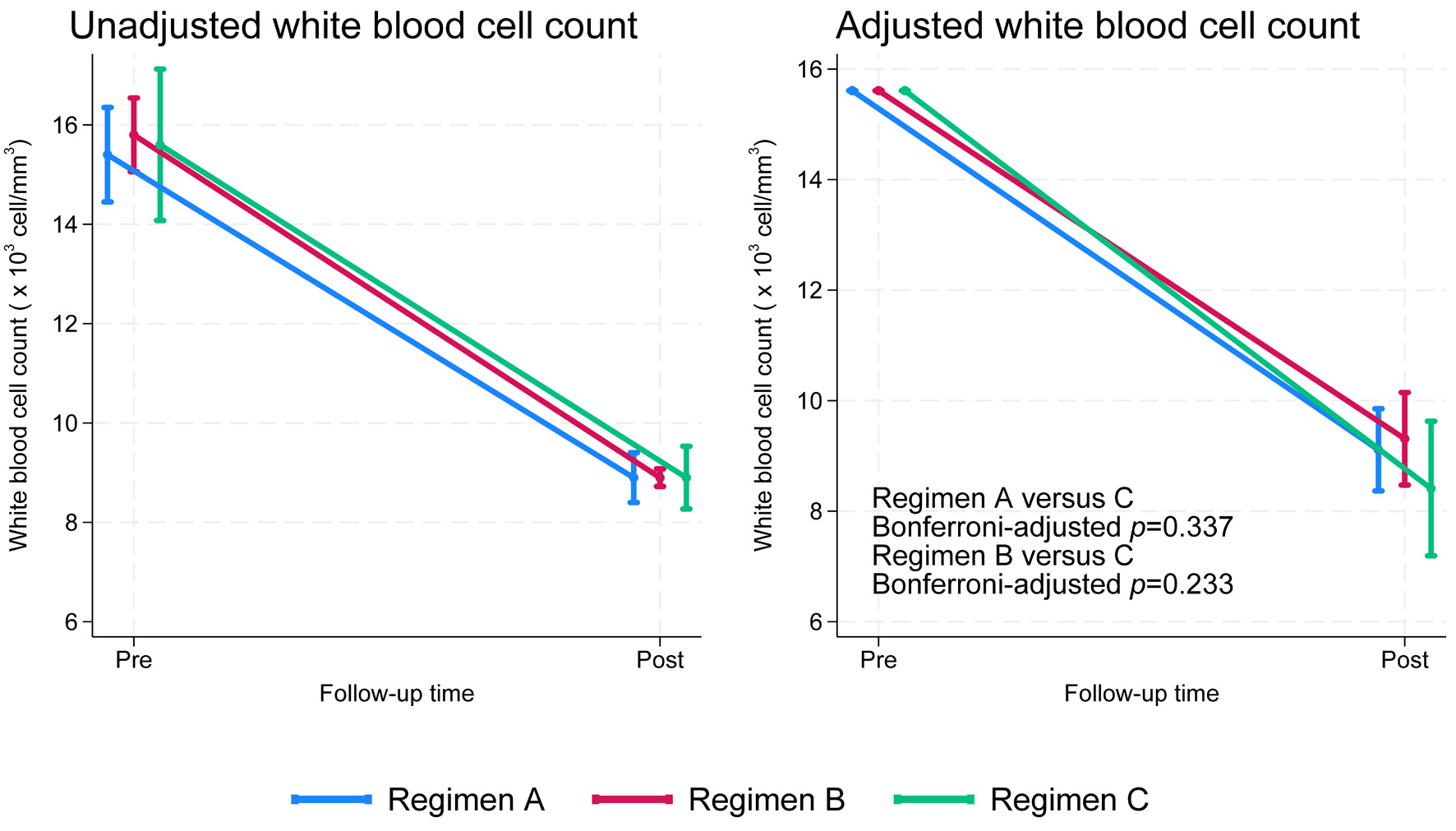 Click for large image | Figure 2. Unadjusted and adjusted white blood cell counts over time by treatment regimen (adjusted for age, pre-treatment white blood cell count, and pre-treatment percentage of polymorphonuclear neutrophils). |
 Click to view | Table 3. White Blood Cell Reduction at 3-Day Follow-Up After Quantile Regression Analysis With Inverse Probability of Treatment Weighting |
 Click to view | Table 4. Pairwise Comparisons of WBC Reduction Between Antibiotic Regimens Using Wald’s Test With Bonferroni-Adjusted P Values |
Treatment failure, as defined in the endpoint section, occurred most frequently in regimen A, followed by B and C, although the differences were not statistically significant. Importantly, no failures led to hospitalization, and no 30-day mortality was observed in this study (Table 5).
 Click to view | Table 5. Secondary Clinical Outcomes Across the Three Antibiotic Regimens |
| Discussion | ▴Top |
In our cohort, all three regimens were associated with a marked reduction in WBC count from baseline to follow-up. Although the slope of decline appeared steeper for regimen C (green line), statistical testing demonstrated no significant difference across groups (global test, P = 0.484). After Bonferroni correction for multiple comparisons, the pairwise differences between regimen C and regimen A (P = 0.337) and between regimen C and regimen B (P = 0.233) remained non-significant. These findings indicate that, despite visual differences in trajectories, the overall WBC reductions achieved by each regimen were comparable.
The OPAT protocol originated in the 1970s in the USA for pediatric patients with cystic fibrosis [2] and was later adopted in countries such as Canada [15], the UK [16], and Australia [17]. In these high-income, highly educated settings, OPAT has been primarily delivered as self-administered therapy, often used for de-escalation after hospital admission or for patients requiring long-term antibiotics for chronic infections. In Asia, Singapore was the first to adopt OPAT in 2012 [18], followed by Japan [19], Malaysia [20], and Thailand [21], where its use has also centered on de-escalation after admission but has remained largely confined to hospital-based or affiliated healthcare facilities.
Regimen A, consisting of IV ceftriaxone as a single initial dose, was evaluated in the COPY-ED study [22], the only published work with a protocol comparable to this regimen in uncomplicated urinary tract infection (UTI). In this study, 57.8% (492/851) of patients received an initial IV antibiotic, most commonly ceftriaxone (86.8%), prior to discharge. The primary analysis compared subsequent oral cephalosporins with oral fluoroquinolones or trimethoprim-sulfamethoxazole, showing clinical cure rates of approximately 95% in both groups without a significant difference.
Regimen B, consisting of once-daily IV ceftriaxone for 3 days, has been shown to be effective for uncomplicated UTI [23] and skin-soft tissue infection (SSTI) [24]. However, in these studies patients were initially admitted to the hospital and, after being classified as low-risk, were subsequently managed with OPAT using ceftriaxone once daily for 3 days, as hospital-based, followed by scheduled clinical follow-up.
Regimen C, consisting of IV ceftriaxone plus oral doxycycline, has primarily been studied in hospitalized patients with community-acquired pneumonia (CAP) [25] providing empirical coverage for Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, as well as atypical pathogens such as Mycoplasma spp. and Chlamydia spp. It has also been applied in hospitalized patients with suspected tropical infections, including Rickettsia spp., Leptospira spp., and pathogens causing enteric fever [26-28].
Our study design differs from most previous reports. Rather than beginning with a wide range of diagnoses, we first identified conditions that could be managed with a uniform treatment approach: IV ceftriaxone as a single dose, IV ceftriaxone once daily for 3 days, or IV ceftriaxone combined with oral doxycycline during the same 3-day course. These regimens were selected for their feasibility in a hospital-based program, once-daily administration, residence within 10 km of the hospital, and reliable follow-up. To ensure patient safety, we applied a strict protocol in which all five components had to be fulfilled before initiating OPAT. Because the choice of regimen often fell into a gray zone depending on physician preference, we used IPTW and quantile regression analysis to minimize confounding by indication, thereby reflecting real-world practice.
This study may represent one of the earliest investigations of an ED-initiated, hospital-based OPAT protocol in Thailand, an area in which research remains scarce. We hypothesize that establishing evidence in this domain could allow the approach to be generalized to other ED diagnoses, such as uncomplicated UTI, SSTI, and infectious diarrhea, thereby reducing inpatient workload, preventing unnecessary hospital admissions, lowering the risk of nosocomial infection, and improving patient satisfaction, as many patients prefer to avoid hospitalization.
Limitations
First, due to the uniform treatment approach in this study, generalization to other infections may be limited. At Lampang Hospital, SSTIs are often caused by mixed organisms, including anaerobes. For very mild cases, oral amoxicillin-clavulanic acid (AMC) is usually prescribed, while patients with leukocytosis typically receive AMC 1 g IV every 8 h. This dosing schedule requires hospital visits three times daily, making OPAT impractical and inpatient admission more convenient for patients.
Second, although the adjusted analysis showed a consistent downward trajectory in WBC across all regimens, the comparisons between regimen C and the others did not reach statistical significance. This lack of significance is most likely attributable to the study being markedly underpowered, as demonstrated by post hoc power analyses showing very low power (< 10%) due to small sample sizes. In particular, the green trajectory for regimen C after adjustment suggests a potential signal that could not be confirmed under the current study design. Therefore, the absence of statistical significance should not be interpreted as absence of effect. To clarify these findings, future multicenter studies with larger cohorts are warranted to provide adequate power and more robust conclusions.
Third, no definitive microbiological diagnosis was established, as patients frequently presented with undifferentiated or non-specific symptoms. Hemocultures and serological tests (IgM, IgG) were not routinely performed because of their high cost and low diagnostic yield. For example, even among hospitalized patients at our center, positive blood culture results are obtained in fewer than 10% of cases despite an annual expenditure of approximately 13 million Thai baht (≈ 350,000 USD), not including the costs of serological assays. In this context, particularly in resource-limited settings, a treatment-based approach may be more practical than extensive pathogen identification.
Finally, this was a single-center study, which may limit the generalizability of our findings to other healthcare settings. The follow-up period was relatively short, focusing on early WBC reduction and immediate clinical response, without evaluating longer-term outcomes such as recurrence or readmission. Although IPTW was applied to adjust for measured confounders, residual confounding from unmeasured factors cannot be excluded.
Conclusion
This study is among the first to evaluate an ED-initiated, hospital-based OPAT protocol in Thailand. Short-course IV ceftriaxone regimens appeared feasible and showed consistent WBC reduction, though statistical significance was limited by small sample size. Larger multicenter studies are needed to confirm these findings.
The authors extend their gratitude to the residents, staff, and nurses of the Department of Emergency Medicine, Lampang Hospital, for their valuable support. We also appreciate the use of ChatGPT (OpenAI) in assisting with the translation and refinement of the English manuscript.
Financial Disclosure
The study was supported by the Lampang Medical Education Center.
Conflict of Interest
The authors reported no contents in the article as conflict of interest.
Informed Consent
Informed consent was waived due to the observational nature of the study.
Author Contributions
Conceptualization: Woraphat Tumporn, Thanin Lokeskrawee, Suppachai Lawanaskol, and Jayanton Patumanond. Data curation: Woraphat Tumporn. Formal analysis: Natthaphon Pruksathorn, Suwapim Chanlaor, Woraphat Tumporn, Wanwisa Bumrungpagdee, Chawalit Lakdee, Thanin Lokeskrawee, Suppachai Lawanaskol, and Jayanton Patumanond. Methodology and supervision: Thanin Lokeskrawee, Suppachai Lawanaskol, and Jayanton Patumanond. Writing - original draft: Woraphat Tumporn and Thanin Lokeskrawee.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AMC: amoxicillin-clavulanic acid; ANOVA: one-way analysis of variance; CAP: community-acquired pneumonia; CERT No.: certificate number; °C: Celsius; 95% CI: 95% confidence interval; ED: emergency department; EDOU: emergency department observation unit; IgG: immunoglobulin G; IgM: immunoglobulin M; IPTW: inverse probability of treatment weighting; IQR: interquartile range; IV: intravenous; kg/m2: kilogram per square meter; mean ± SD: mean ± standard deviation; n (%): number (percentage); NEWS: National Early Warning Score; OPAT: outpatient parenteral antimicrobial therapy; P value: probability value; PMN: polymorphonuclear neutrophil; pre-Tx: pre-treatment; SE: standard error; SSTI: skin-soft tissue infection; TCTR: Thai Clinical Trials Registry; UA: urinalysis; UTIs: urinary tract infections; WBC: white blood cell
| References | ▴Top |
- Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med. 2017;45(9):1443-1449.
doi pubmed - Rucker RW, Harrison GM. Outpatient intravenous medications in the management of cystic fibrosis. Pediatrics. 1974;54(3):358-360.
pubmed - Mohammed SA, Roberts N, Nicolas D, Unwin S, Cotta M, Roberts JA, Sime FB. Implementation of outpatient parenteral antimicrobial therapy program in the contemporary health care system: A narrative review of the evidence. J Infect Public Health. 2025;18(10):102938.
doi pubmed - Thomnoi T, Santimaleeworagun W. The community hospital-based parenteral antimicrobial therapy (COHPAT) in Thailand: The experience of a model to continuously treat bacterial infections at Khlongluang Hospital. Thai Bull Pharm Sci. 2021;16(2):19-31.
- CDC. FluView [Internet]. Atlanta: CDC; 2025. U.S. Influenza Surveillance: Purpose and Methods; 2023 [cited Sep 15, 2024]. Available from: https://www.cdc.gov/fluview/overview/index.html.
- Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017;45(3):299-307.
doi pubmed - Cerny J, Rosmarin AG. Why does my patient have leukocytosis? Hematol Oncol Clin North Am. 2012;26(2):303-319.
doi pubmed - Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP, 2017.
- Scott LJ, Redmond NM, Garrett J, Whiting P, Northstone K, Pullyblank A. Distributions of the National Early Warning Score (NEWS) across a healthcare system following a large-scale roll-out. Emerg Med J. 2019;36(5):287-292.
doi pubmed - Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670-1677.
doi pubmed - Wald DA. Undifferentiated and differentiated patients. In: Wald DA, editor. Emergency medicine clerkship primer: A manual for medical students. Illinois: Clerkship Directors in Emergency Medicine; 2011. p. 23-30.
- Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. Hoboken (NJ): Wiley; 2013.
- Powers D, Xie Y. Statistical methods for categorical data analysis. 2nd ed. Bingley: Emerald; 2008.
- Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277.
doi pubmed - Yan M, Lam PW, Andany N, Chan AK, Leis JA, Mubareka S, Simor AE, et al. Assessing the utilization and impact of a newly established outpatient parenteral antimicrobial therapy (OPAT) program. J Assoc Med Microbiol Infect Dis Can. 2020;5(2):70-76.
doi pubmed - Gilchrist M, Barr D, Drummond F, Muir A, Williams J, Scriven J, Snape S, et al. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: findings from the BSAC National Outcomes Registry (2015-19). J Antimicrob Chemother. 2022;77(5):1481-1490.
doi pubmed - Subedi S, Looke DF, McDougall DA, Sehu MM, Playford EG. Supervised self-administration of outpatient parenteral antibiotic therapy: a report from a large tertiary hospital in Australia. Int J Infect Dis. 2015;30:161-165.
doi pubmed - Chan M, Ooi CK, Wong J, Zhong L, Lye D. Role of outpatient parenteral antibiotic therapy in the treatment of community acquired skin and soft tissue infections in Singapore. BMC Infect Dis. 2017;17(1):474.
doi pubmed - Hase R, Yokoyama Y, Suzuki H, Uno S, Mikawa T, Suzuki D, Muranaka K, et al. Review of the first comprehensive outpatient parenteral antimicrobial therapy program in a tertiary care hospital in Japan. Int J Infect Dis. 2020;95:210-215.
doi pubmed - Lim AH, Ab Rahman N, Nor ANM, Misnan NA, Tok PSK. Evaluation of the outpatient parenteral antimicrobial therapy (OPAT) service in Malaysian public hospitals: a mixed-methods study protocol. BMJ Open. 2025;15(6):e100292.
doi pubmed - Thomnoi T, Komenkul V, Prawang A, Santimaleeworagun W. Impact of pharmacist-led implementation of a community hospital-based outpatient parenteral antimicrobial therapy on clinical outcomes in Thailand. Antibiotics (Basel). 2022;11(6).
doi pubmed - Koehl J, Spolsdoff D, Negaard B, Lewis A, Santiago R, Krenz J, Polotti A, et al. Cephalosporins for outpatient pyelonephritis in the emergency department: COPY-ED study. Ann Emerg Med. 2025;85(3):240-248.
doi pubmed - Elajouz B, Dumkow LE, Worden LJ, VanLangen KM, Jameson AP. Three-day ceftriaxone versus longer durations of therapy for inpatient treatment of uncomplicated urinary tract infection. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e171.
doi pubmed - Yadav K, Suh KN, Eagles D, Thiruganasambandamoorthy V, Wells GA, Stiell IG. Evaluation of an emergency department to outpatient parenteral antibiotic therapy program for cellulitis. Am J Emerg Med. 2019;37(11):2008-2014.
doi pubmed - Ingram PR, Cerbe L, Hassell M, Wilson M, Dyer JR. Limited role for outpatient parenteral antibiotic therapy for community-acquired pneumonia. Respirology. 2008;13(6):893-896.
doi pubmed - Wangrangsimakul T, Althaus T, Mukaka M, Kantipong P, Wuthiekanun V, Chierakul W, Blacksell SD, et al. Causes of acute undifferentiated fever and the utility of biomarkers in Chiangrai, northern Thailand. PLoS Negl Trop Dis. 2018;12(5):e0006477.
doi pubmed - Luvira V, Silachamroon U, Piyaphanee W, Lawpoolsri S, Chierakul W, Leaungwutiwong P, Thawornkuno C, et al. Etiologies of acute undifferentiated febrile illness in Bangkok, Thailand. Am J Trop Med Hyg. 2019;100(3):622-629.
doi pubmed - Wodniak NR, Bhengsri S, Skaggs B, Uttayamakul S, Sawatwong P, Sangwichian O, Gregory CJ, et al. Demographic and clinical factors associated with bacterial or nonbacterial etiologies of acute undifferentiated febrile illness: findings from a 3-year observational study in Thailand, 2017-2020. Am J Trop Med Hyg. 2024;111(3):650-660.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.