| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 10, October 2025, pages 582-594
The Healing Effect of Aged Garlic Extract on Acetic Acid and 5-Fluorouracil-Induced Oral Mucositis in Mice
Keisuke Kawasakia, Koji Haradab, d, Tarannum Ferdousb, Keishiro Isayamac, Kenji Watanabec, Yoichi Mizukamic, Katsuaki Mishimaa
aDepartment of Oral and Maxillofacial Surgery, Yamaguchi University Graduate School of Medicine, Ube 755-8505, Japan
bDepartment of Nursing, Faculty of Health Sciences, Hiroshima Cosmopolitan University, Hiroshima 734-0014, Japan
cCenter for Gene Research, Science Research Center, Yamaguchi University, Ube 755-8505, Japan
dCorresponding Author: Koji Harada, Department of Nursing, Faculty of Health Sciences, Hiroshima Cosmopolitan University, Hiroshima 734-0014, Japan
Manuscript submitted August 2, 2025, accepted October 13, 2025, published online October 29, 2025
Short title: AGE May Exert a Healing Effect on Oral Mucositis
doi: https://doi.org/10.14740/jocmr6341
| Abstract | ▴Top |
Background: This preliminary study investigated the effect of aged garlic extract (AGE) on acetic acid (AA) and/or 5-fluorouracil (5-FU)-induced oral mucositis in tumor-bearing mice, and whether AGE affects the antitumor activity of 5-FU.
Methods: There were four mouse groups: control, AA, AA + 5-FU, and AA + 5-FU + AGE. Mouse squamous cell carcinoma cells (SCCVII) were used to develop tumors in mice, except for the control group. Oral mucositis was induced in tumor-bearing mice by intraperitoneal injection with 5-FU (18 mg/kg) for 9 days and/or topical application of 50% AA to the dorsal tongue for 1 day. Mucositis was treated with AGE (2.0 g/kg/day) for 10 days in AA + 5-FU + AGE group, while the other groups received saline (0.2 mL/day). The wound healing and antitumor effects of AGE were examined. Whole transcriptome analysis and ingenuity pathways analysis (IPA) of the tongue and tumor samples were used to investigate the mechanisms behind the wound healing and antitumor effects of AGE.
Results: Body weight was increased significantly in AA + 5-FU + AGE group compared to AA + 5-FU group. Moreover, tumor volume was significantly decreased in AA + 5-FU + AGE group than that in the other groups. In AA + 5-FU group, toluidine blue-positive area (wound area) in the tongue was the largest, and the size and weight of the salivary glands were decreased compared to other groups. In contrast, wound area was significantly reduced, and the size and weight of the salivary glands were increased in AA + 5-FU + AGE group compared to AA + 5-FU group. Therefore, AGE treatment could heal tongue ulcers and salivary gland damage in AA + 5-FU + AGE group. Whole transcriptome analysis and IPA data suggested that AGE could heal 5-FU-induced oral mucositis by promoting normal cell differentiation and keratinization, and it may also enhance the antitumor effects of 5-FU through the activation of B cells in mouse tumors.
Conclusion: AGE could alleviate AA and 5-FU-induced oral mucositis in mice while potentially enhancing the antitumor activity of 5-FU. Therefore, AGE might be useful in the treatment of oral mucositis in cancer patients receiving 5-FU-based therapies.
Keywords: Aged garlic extracts; Oral mucositis; 5-fluorouracil; Acetic acid; Salivary glands
| Introduction | ▴Top |
Oral mucositis is one of the major adverse reactions of chemotherapy and radiotherapy, which increases the duration of hospitalization and cost of care, and negatively impacts the survival of cancer patients [1, 2]. However, there is no effective treatment for severe oral mucositis until now. Briefly, it is one of the unmet medical needs of patients suffering from cancer, including oral squamous cell carcinoma (OSCC). Once severe oral mucositis occurs, the cancer patients suffer from a wide range of complications including acute oral pain, infection, bleeding, bacteremia, sepsis, salivary gland atrophy, and xerostomia. These symptoms are associated with salivary gland dysfunction and poor oral hygiene, which may result in poor nutritional intake, body weight loss, and overall health deterioration in cancer patients, especially in immunosuppressed patients [1-5]. Anticancer agents may damage basal epithelium and mucosal lining in the oral cavity, oropharynx, gut and skin, as well as the stem cells [6-9]. Salivary gland functions and saliva volume also play an important role in maintaining the health and functionality of the oral cavity and oropharynx [3]. Saliva provides antimicrobial, lubricating, and protective effects through numerous proteins and peptides, including immunoglobulin A (IgA), lactoferrin, lysozymes, defensins, and mucins [10]. It is a well-established fact that chemotherapeutic agents that are commonly used in OSCC treatments including 5-fluorouracil (5-FU), taxanes, and platinum derivatives injure salivary gland tissues, and decrease saliva secretion volume as well as saliva proteins, which might contribute to the occurrence and severity of oral mucositis [3, 11-13]. The available therapies for the prevention or treatment of chemotherapy-induced mucositis have shown limited efficacy [14-17]. Most of these treatments are not designed to protect salivary glands and their functions from the adverse effects of chemotherapy. Therefore, finding new strategies to alleviate the side effects of anticancer drugs and to develop novel and effective therapies for mucositis is urgently needed.
Aged garlic extract (AGE) is one of the widely used garlic-based products that are extensively studied and commercially available. It is prepared by soaking fresh and chopped garlic with aqueous ethanol for more than 10 months at room temperature, which is necessary to increase the levels of beneficial compounds and antioxidants in the end product [18]. AGE has a wide range of health benefits because of its anti-oxidation [19, 20], anti-inflammation [21-23], wound healing [24-27], immunomodulation [28-30], and anticarcinogenic effects [31]. AGE is also effective against periodontal diseases as reported in several clinical studies [32, 33]. We assumed that the anti-oxidation, anti-inflammation, and wound healing effects of AGE might be useful in alleviating oral mucositis, and in protecting salivary glands and their functions.
In this preliminary study, the healing effects of AGE against acetic acid (AA) and 5-FU-induced oral mucositis were investigated. In addition, we tried to understand whether AGE contributes to 5-FU-induced tumor inhibition.
| Materials and Methods | ▴Top |
Animals
Ten-week-old female C3H/HeJJcl mice (n = 16) were obtained from CLEA Japan Inc. (Tokyo, Japan). Mice were housed in a controlled pathogen-free environment in accordance with the Guidelines for Animal Experimentation of Yamaguchi University, Ube, Japan. They were adapted in that environment for 1 week before the experiment and were provided with food and water ad libitum. All animal experiments were approved by the Animal Experimentation Committee of Yamaguchi University (approval no. 55-024), and conformed to all the guidelines for animal experimentation outlined by the committee.
Development of tumors in nude mice
Sixteen mice were randomly allocated in four groups without any bias. Mice in all groups were of same age and sex, and they had similar average body weight at the beginning of the study. A mouse squamous cell carcinoma cell line, SCCVII was used to develop tumors in 11-week-old female C3H/HeJJcl mice. Tumors were developed in 12 mice only and the other four mice were without any tumor (control group). Mice were anesthetized with ether, and then SCCVII cells (1 × 105) were suspended in 0.1 mL of serum-free DMEM medium and injected subcutaneously into the back region of each mouse using a 27-gauge needle. When the tumors reached about 1,000 mm3 in volume, these mice were used for the oral mucositis experiment. The tumor volume was measured every 2 days until the end of the experiment and was calculated as 0.5 × length × width2.
Induction of oral mucositis on mice by 5-FU administration and AA topical application
Control group (n = 4) did not bear any tumors. Twelve tumor-bearing mice were randomly divided into three treatment groups (AA group, AA + 5-FU group, and AA + 5-FU + AGE group; n = 4) on day 0 (time of grouping is referred to as day 0). Mice in all treatment groups had similar average body weight and similar average tumor volume (1,000 mm3). Figure 1 shows the experimental schedule. Oral mucositis was induced in all groups (except for the control) by the topical application of 50% AA on the dorsal tongue, while AA + 5-FU and AA + 5-FU + AGE groups were also treated with intraperitoneal injection of 5-FU (18 mg/kg/day, Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan). AA group and control group received saline (0.2 mL/day) intraperitoneally instead of 5-FU. 5-FU or saline were administered for 9 days (from day 0 to day 8), and AA was applied for 30 s for 1 day only (on day 6). As a treatment for oral mucositis, AA + 5-FU + AGE group received AGE (2.0 g/kg/day; Wakunaga Pharmaceutical Co., Ltd., Tokyo, Japan) orally for 10 days (day 0 to day 9), while the control, AA, and AA + 5-FU groups received saline orally (0.2 mL/day) for the same period.
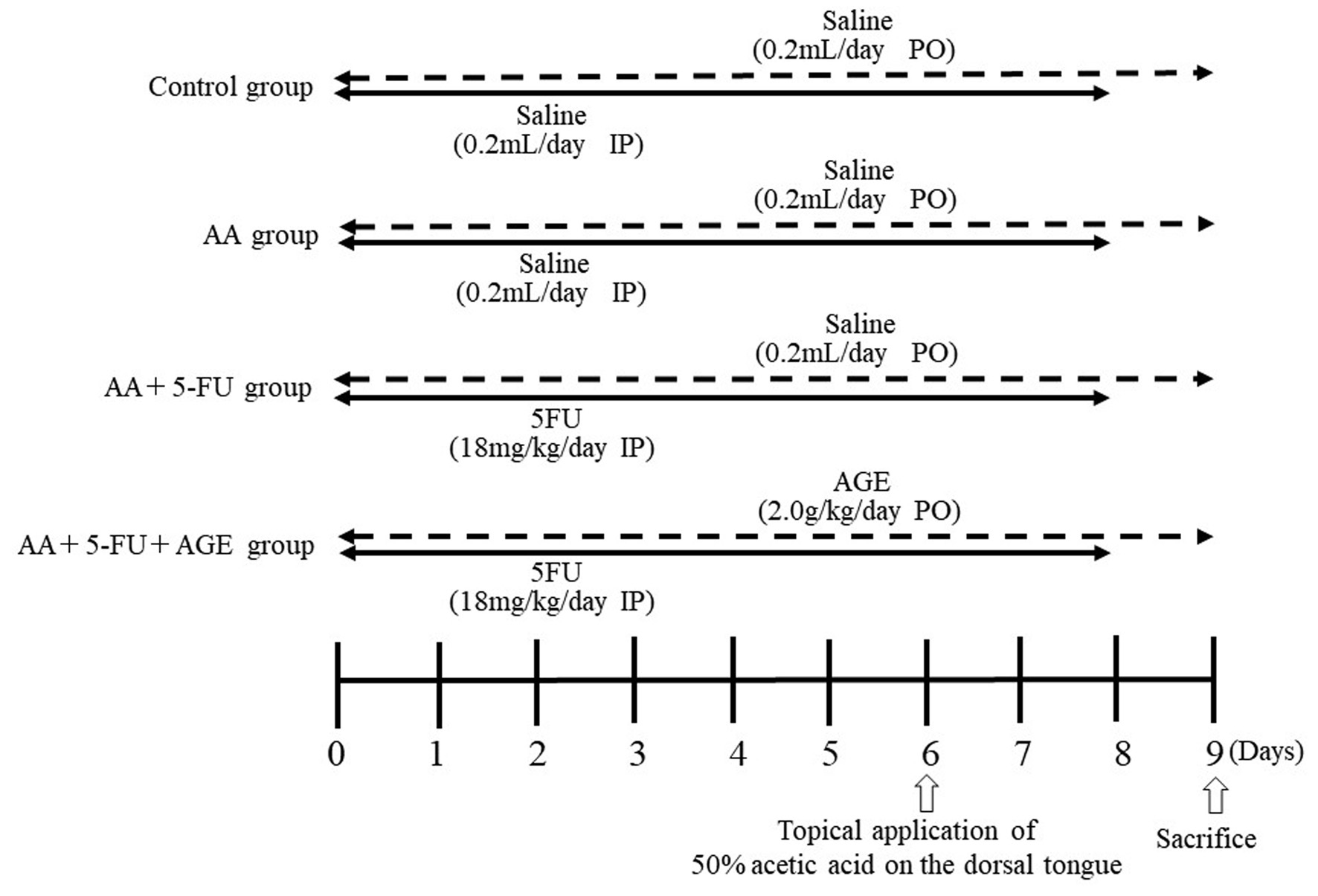 Click for large image | Figure 1. Experimental protocol and schedule. Three groups (AA, AA + 5-FU, and AA + 5-FU + AGE) are SCCVII tumor-bearing mice (n = 4), and the other group is control without any tumor (n = 4). Oral mucositis was induced in AA + 5-FU and AA + 5-FU + AGE groups by intraperitoneal injection of 5-FU (18 mg/kg/day) for 9 days (from day 0 to day 8); while the control and AA groups received saline (0.2 mL/day) intraperitoneally for the same period. Mice of all groups except for the control received 50% AA on the dorsal tongue on day 6. As a treatment for oral mucositis, AA + 5-FU + AGE group received AGE (2.0 g/kg/day) orally for 10 days (day 0 to day 9), while the control, AA, and AA + 5-FU groups received saline orally (0.2 mL/day) for the same period. All mice were sacrificed on day 9. AA: acetic acid; AGE: aged garlic extract; 5-FU: 5-fluorouracil. |
Body weight was measured from day 0 to day 9 before administration of 5-FU, saline or AGE. The antitumor effect of 5-FU against tumor growth was monitored, and tumor volume at the inoculation site was measured regularly. The humane endpoint of the experiment was decided as maximum tumor size 3,500 mm3 and < 40% loss of body weight. The experiment was terminated on day 9 and all the mice were sacrificed by carbon dioxide overdose. Tumors, tongues, and salivary glands (the submandibular gland and sublingual gland) were collected, measured, and weighed on day 9 soon after sacrifice. The volume (mm3) of the salivary glands was calculated by the following formula: 0.4 × length (L) × width (W2) [34].
Toluidine blue staining of the wounded tongues and assessment of oral mucositis
The tongues were excised and stained with 1% toluidine blue dissolved in 10% AA for 1 min in order to visualize and estimate the size of the ulcerative lesions or wounds on the tongue surface. After staining, the tongues were washed repeatedly with 1% AA until all excess dye was removed. The toluidine blue-stained wounds were measured manually with a ruler at first, and the wound area of each tongue was calculated by the following formula: area (mm2) = length (L) × width (W). Then, tongue images were acquired with a digital camera, and the toluidine blue-stained areas were measured and re-calculated by imageJ 1.54m software (National Institutes of Health, Rockville, MD, USA; https://imagej.net/ij/) to confirm our data.
Hematoxylin and eosin (H&E) staining of the tongues and histological examination
After the tongue samples were fixed in 4% neutral buffered paraformaldehyde at room temperature for 24 h, samples were embedded in paraffin. The 4 µm-thick tissue sections were subjected to H&E staining. The tissue sections were immersed in xylene followed by graded ethanol (100-70%) and then washed with tap water at room temperature. Then, they were immersed in hematoxylin for 10 min, 1% acid-ethanol for 30 s, 1% ammonia water for 30 s, and thoroughly washed under running tap water. The sections were then treated with eosin for 1 min, and were subsequently dehydrated in graded ethanol (70-100%) and xylene. After clearing the section with Histoclear (National Diagnostics, Atlanta GA, USA), the slides were mounted with glass coverslips using DPX mounting medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Then, the wounds or ulcerative lesions of each tongue were observed and evaluated under a fluorescence microscope (BX51; Olympus Corporation, Tokyo, Japan).
Whole transcriptome analysis
Tongue and tumor samples were frozen, and then used for whole transcriptome analysis. Total RNA was isolated from samples using the Maxwell RSC simply RNA tissue kit (Promega, Madison, WI, USA). The quality of RNA was examined with a Tapestation 4200 (Agilent Technologies, Inc., Santa Clara, CA, USA) using a high sensitivity RNA kit (Agilent Technologies, Inc.) after the concentrations were determined by Nanodrop one (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The RNA integrity number values were more than 7.6 in all samples. Using 100 ng of total RNA, poly(A) RNA was extracted and fragmented using the NEBNext poly(A) mRNA magnetic isolation module (#E7490; New England BioLabs or NEB, Ipswich, MA, USA) and the NEBNext Ultra II directional RNA library prep kit for Illumina (#E7760; NEB). Briefly, poly(A) RNA was isolated using oligo dT beads, and was fragmented in the presence of NEBNext first strand synthesis reaction buffer and NEBNext random primers by incubation at 94 °C for 15 min. The fragmented poly(A) RNA was reverse transcribed using the NEBNext first strand synthesis enzyme mix to synthesize single-stranded cDNA. Double-stranded cDNA was then synthesized using the NEBNext second strand synthesis enzyme mix, and the cDNA was purified using AMPure XP (#A63882, Beckman Coulter, Inc. Pasadena, CA, USA). Adaptor sequences were then inserted to cDNA using the NEBNext adaptor (#E6448, NEB), and were purified using AMPure XP. The cDNA was amplified by PCR using a c1000 Touch thermal cycler (Bio-Rad Laboratories, Inc. Hercules, CA, USA). During the amplification, barcode sequences were added to the samples using NEBNext multiplex oligos for Illumina (#E6448, NEB). The amplified library was then purified using AMPure XP. The concentration of the library was measured using a Qubit 2.0 fluorometer (Thermo Fisher Scientific), and the length distribution of the library was analyzed using a Tapestation 4200 D1000 (#5067-5582, Agilent Technologies). The libraries were sequenced on an Illumina NovaSeq 6000 sequencer (Illumina, Inc. San Diego, CA, USA) with an Illumina NovaSeq 6000 S1 reagent kit ver1.5 (100 cycle, Illumina). More than 50 million reads in each sample were detected in the reaction and were trimmed and mapped with the mouse reference genome GRCm38 release-94 using CLC Genomics Workbench software 22.0.1 (Qiagen, Santa Clarita, CA, USA). The mapping ratio in each sample was more than 97%.
Ingenuity pathway analysis (IPA)
Co-relation analysis was conducted with Prism (ver.10.2.3; GraphPad Software, Boston, MA, USA). The gene sets that demonstrated significantly increased or decreased expression in the co-relation analysis were then examined by network analysis using the IPA software (version 8.6, Qiagen). The IPA software revealed the molecular and cellular functions of the dataset members as well as the canonical pathways represented in the dataset. The IPA software is derived from a vast amount of molecular interactions reported in the literature and the software is updated weekly. The IPA uses a Fisher’s exact test to determine whether the differentially expressed genes are significantly related to pathways compared with the whole ingenuity knowledge base.
Statistical analysis
All data are expressed as mean value ± standard error of the mean (SEM). Data of mouse body weight and tumor volume were compared using two-way analysis of variance (ANOVA) followed by Tukey-Kramer post hoc test for multiple comparison. Data of tongue toluidine blue-stained (wound) area and salivary gland weight were compared using one-way ANOVA followed by Tukey-Kramer post hoc test. Significance was defined as: P < 0.05. All statistical analyses were conducted using StatView v.5.0J software (SAS Institute, Inc., Cary, NC, USA).
| Results | ▴Top |
Effect of AGE on body weight and tumor volume in mice
Although no noticeable difference in body weight or tumor size among different mouse groups was detected to the naked eye (Fig. 2a), mice in AA + 5-FU group had lower body weight and second largest tumors than all other groups (Fig. 2b, c). There was a significant difference in body weight between AA + 5-FU group and control group, but not between AA + 5-FU + AGE group and control group. It seemed like that the body weight loss was somewhat recovered by AGE administration in AA + 5-FU + AGE group (Fig. 2b). Tumor volume was significantly decreased in AA + 5-FU + AGE group compared to the AA + 5-FU group and AA group. Therefore, AGE did not negatively influence the antitumor effect of 5-FU, and it possibly inhibited tumor growth by enhancing the efficacy of 5-FU to some extent (Fig. 2c).
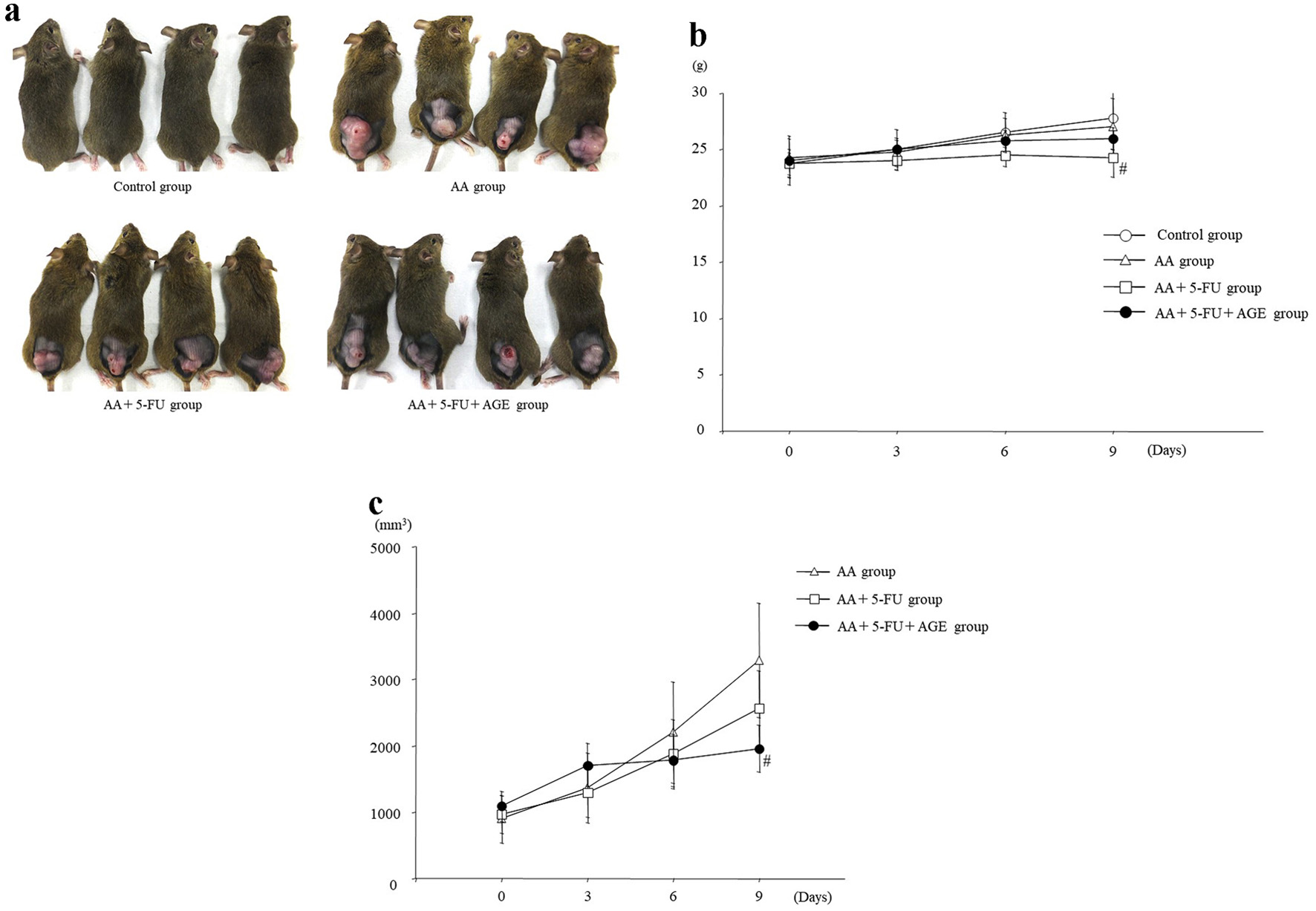 Click for large image | Figure 2. Effect of AGE on body weight and tumor volume in mice. (a) Photos of the control and tumor-bearing mice taken just before sacrifice. (b) Graph showing average body weight (g) of mice in each group. 5-FU treatment caused weight loss in AA + 5-FU group and AA + 5-FU + AGE group compared to the control. However, the average body weight was higher in AA + 5-FU + AGE group than in AA + 5-FU group. Briefly, AGE might have improved mouse health. Mean ± SEM; #P < 0.05 (two-way analysis of variance and Tukey-Kramer post hoc test). (c) Graph showing the effect of 5-FU and/or AGE on tumor growth in different groups. Tumor volume (mm3) was significantly decreased in AA + 5-FU + AGE group compared to the AA + 5-FU group and AA group. AGE did not negatively influence the antitumor effects of 5-FU, probably enhanced it. Mean ± SEM; #P < 0.05 (two-way analysis of variance and Tukey-Kramer multiple post hoc test). AA: acetic acid; AGE: aged garlic extract; 5-FU: 5-fluorouracil; SEM: standard error of the mean. |
Effect of AGE on the wounded area or ulcerative lesions on dorsal tongue
It was difficult to detect and fully examine wounds or mucositis induced by AA topical application and/or 5-FU-administrtion with the naked eye. Without staining, wounded area or ulcerative lesions was not observed clearly in all groups except AA + 5-FU group (Fig. 3a, left). After toluidine-blue staining, wound areas/ulcers on the mouse tongues were clearly visible in all groups (Fig. 3a, right). Tongues of AA + 5-FU group showed strong staining, whereas AA group showed moderate staining. The mucositis/wound (toluidine blue-positive) area in AA + 5-FU + AGE group was reduced compared to all other groups, and it was significantly smaller than that in AA + 5-FU group (Fig. 3a, b). In Fig. 3c, H&E staining of the tongue sections shows erosive or ulcerative lesions on the tongue surface. Extremely atrophic and absent epithelial regions were observed in AA + 5-FU group, which were much larger than those in other groups. In contrast, epithelialization of the dorsal tongue was clearly observed in AA + 5-FU + AGE group as well as in AA group. Therefore, AGE treatment could decrease the erosive or ulcerative lesions on the mouse tongue and promote epithelization of the wound area in AA + 5-FU + AGE group (Fig. 3c). These data indicate that AGE might protect and heal tongues from 5-FU and AA-induced damage.
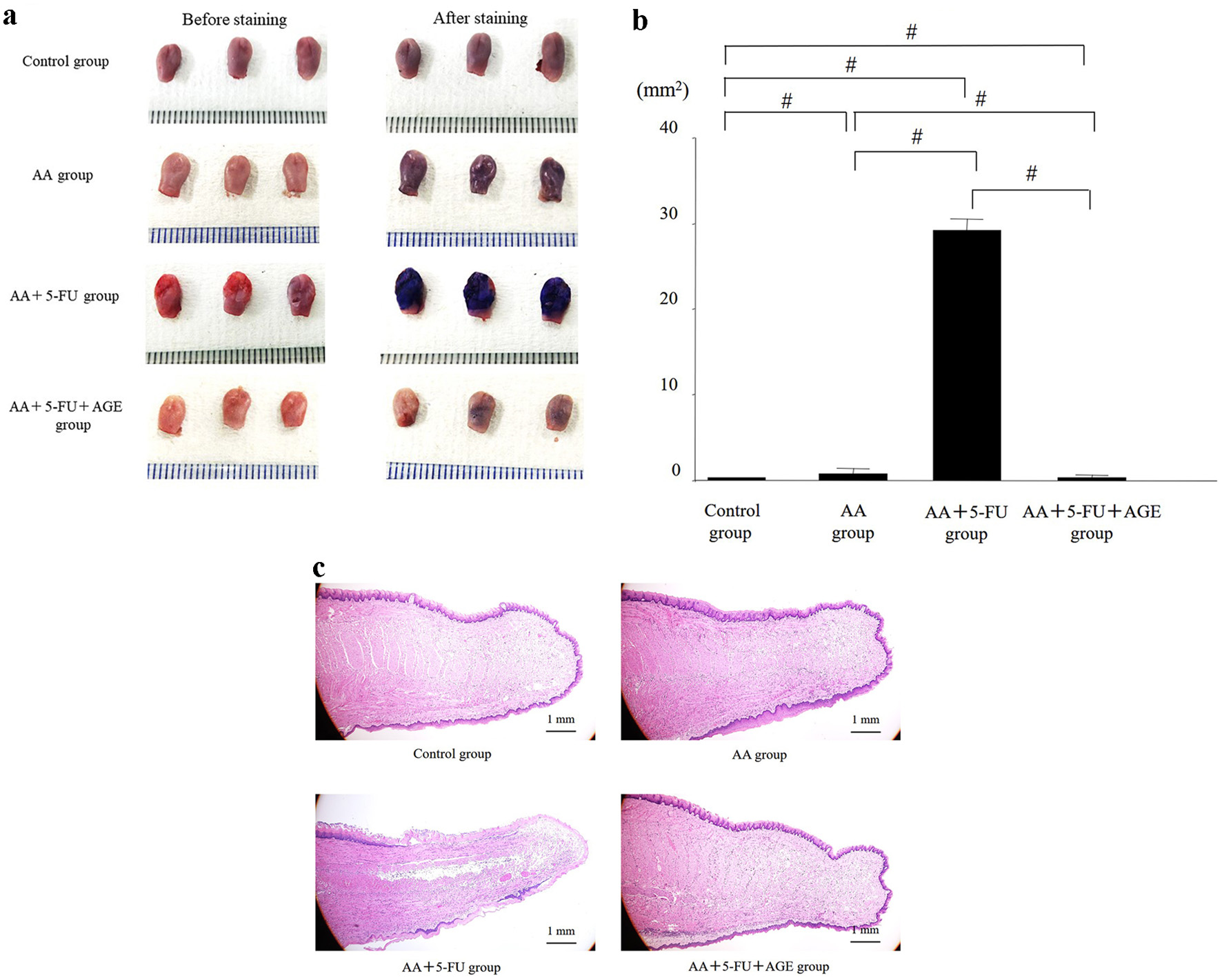 Click for large image | Figure 3. Effect of AGE on the wounded/mucositis area or ulcerative lesions on the mouse dorsal tongue. (a) Photos of the mouse tongues before (left side) and after toluidine blue staining (right side). Toluidine blue stains made wounded areas or ulcers visible. The largest (toluidine blue- positive) wound areas with strong staining were observed in AA + 5-FU group. Mildly stained small wounds were visible on the tongues of AA group. The staining intensity was significantly reduced and the wounds were comparatively smaller in AA + 5-FU + AGE group than those in AA + 5-FU group. (b) Graph showing the effect of AGE on tongue wound/mucositis area (mm2) in different groups. The toluidine blue-positive tongue wound area was the largest in AA + 5-FU group. The wound area in AA + 5-FU + AGE group was significantly smaller compared to all other groups. AGE treatment could heal the wound/ulcer caused by 5-FU and AA. Mean ± SEM; #P < 0.05 (one-way analysis of variance and Tukey-Kramer post hoc test). (c) H&E staining of the tongue sections. Severe damage in the epithelial region was observed in AA + 5-FU group, while epithelial damage was almost absent in AA + 5-FU + AGE group. AGE could protect and heal tongues from 5-FU and AA-induced damage. AA: acetic acid; AGE: aged garlic extract; 5-FU: 5-fluorouracil; H&E: hematoxylin and eosin; SEM: standard error of the mean. |
Effect of AGE on the size and weight of salivary glands in mice
Decreased saliva secretion is associated with oral mucositis in patients receiving cancer chemotherapy [35]. In this study, the size and weight of salivary glands of each group of mice were evaluated. Salivary gland size was smaller in all of the treatment groups (AA, AA + 5-FU, and AA + 5-FU + AGE) compared to the control, especially in AA + 5-FU group. Interestingly, the salivary glands of AA + 5-FU + AGE group were markedly larger than those of AA + 5-FU group (Fig. 4a). In addition, there was no significant difference in the average weight of salivary glands between the control and AA group. The weight of the salivary glands decreased significantly in AA + 5-FU group after 5-FU and AA treatment, which was recovered after AGE treatment as observed in AA + 5-FU + AGE group (Fig. 4b). Therefore, AGE could suppress salivary gland atrophies caused by 5-FU and AA.
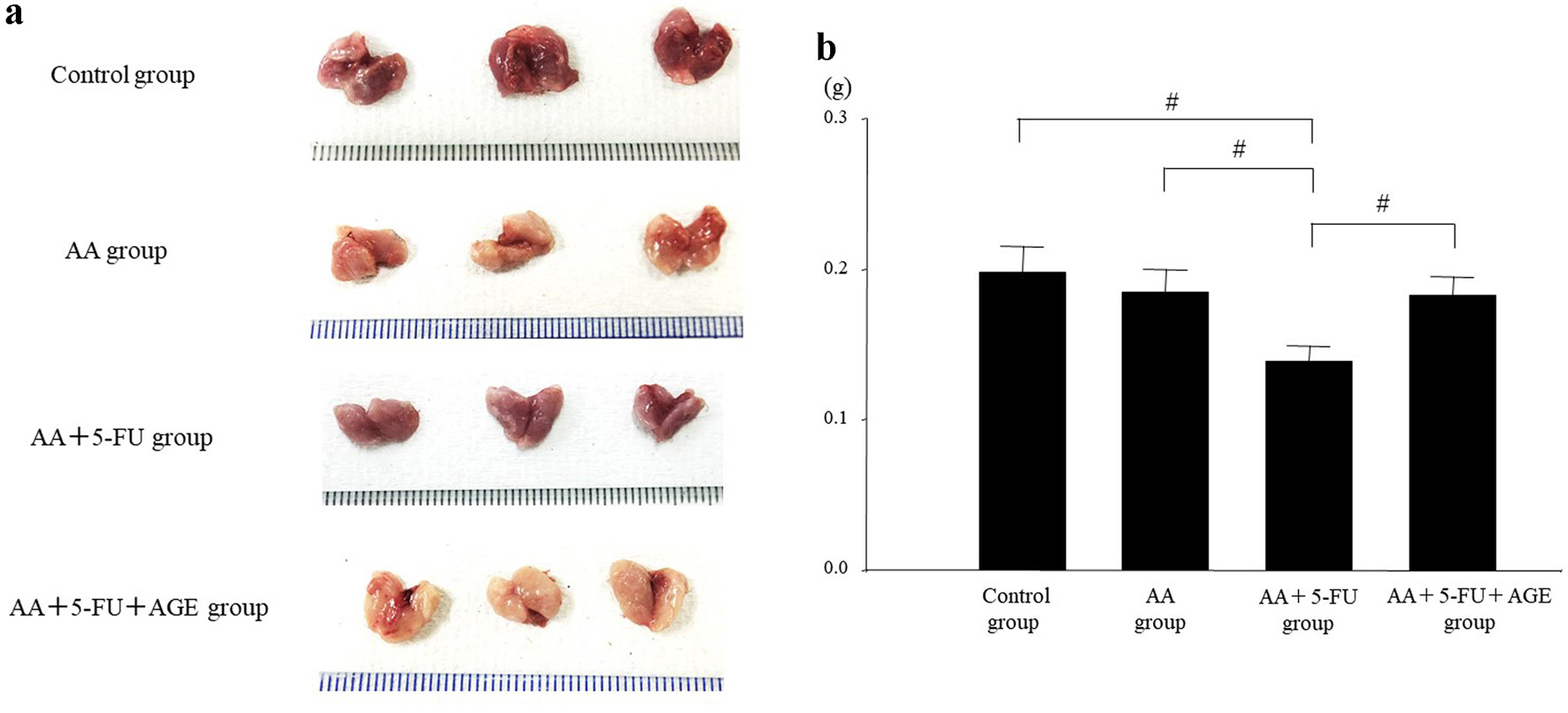 Click for large image | Figure 4. Effect of AGE on the size and weight of salivary glands in mice. (a) Photos of the extracted salivary glands at the time of sacrifice. Salivary gland size was smaller in the all treatment groups (AA, AA + 5-FU, and AA + 5-FU + AGE) compared to the control. The gland size was the smallest in AA + AGE group, while it was increased markedly in AA + 5-FU + AGE group compared to AA + 5-FU group. (b) Graph showing the effect of AGE on salivary gland weight (g) in different groups. There was no significant difference in the average weight of the salivary glands between the control and AA group. Salivary gland weight was significantly decreased in AA + 5-FU group, which was recovered in AA + 5-FU + AGE group after AGE treatment. Mean ± SEM; #P < 0.05 (one-way analysis of variance and Tukey-Kramer post hoc test). AA: acetic acid; AGE: aged garlic extract; 5-FU: 5-fluorouracil; SEM: standard error of the mean. |
Mechanism of action of wound healing and antitumor effect of AGE
To understand the mechanisms behind the wound healing and antitumor effects of AGE, we compared the tongue and tumor samples of AA + 5-FU and AA + 5-FU + AGE groups by whole transcriptome analysis. Next-generation sequencing and IPA were used to search for specific gene groups whose expressions may have changed between these two mouse groups.
The first canonical pathway for tongue samples showed the genes related to the keratinization process, which is important for wound healing (Fig. 5a). An enlarged view of a part of that pathway in Figure 5a (marked by a red square) was shown in Figure 5b. Between AA + 5-FU + AGE and AA + 5-FU groups, the activation of genes related to the keratinization process was observed in AA + 5-FU + AGE group (Fig. 5b). Network analysis showed activation of protein kinase B (PKB) or Akt kinases as well as the subsequent aggregation of serine protease and kallikrein in AA + 5-FU + AGE group (Fig. 5c). Akt kinases regulate cellular survival, and Akt together with serine protease and kallikrein are involved in wound healing and repairing of damaged epidermis. Moreover, in network 3, a noticeable aggregation of keratin-associated proteins was observed in AA + 5-FU + AGE group (Fig. 5d).
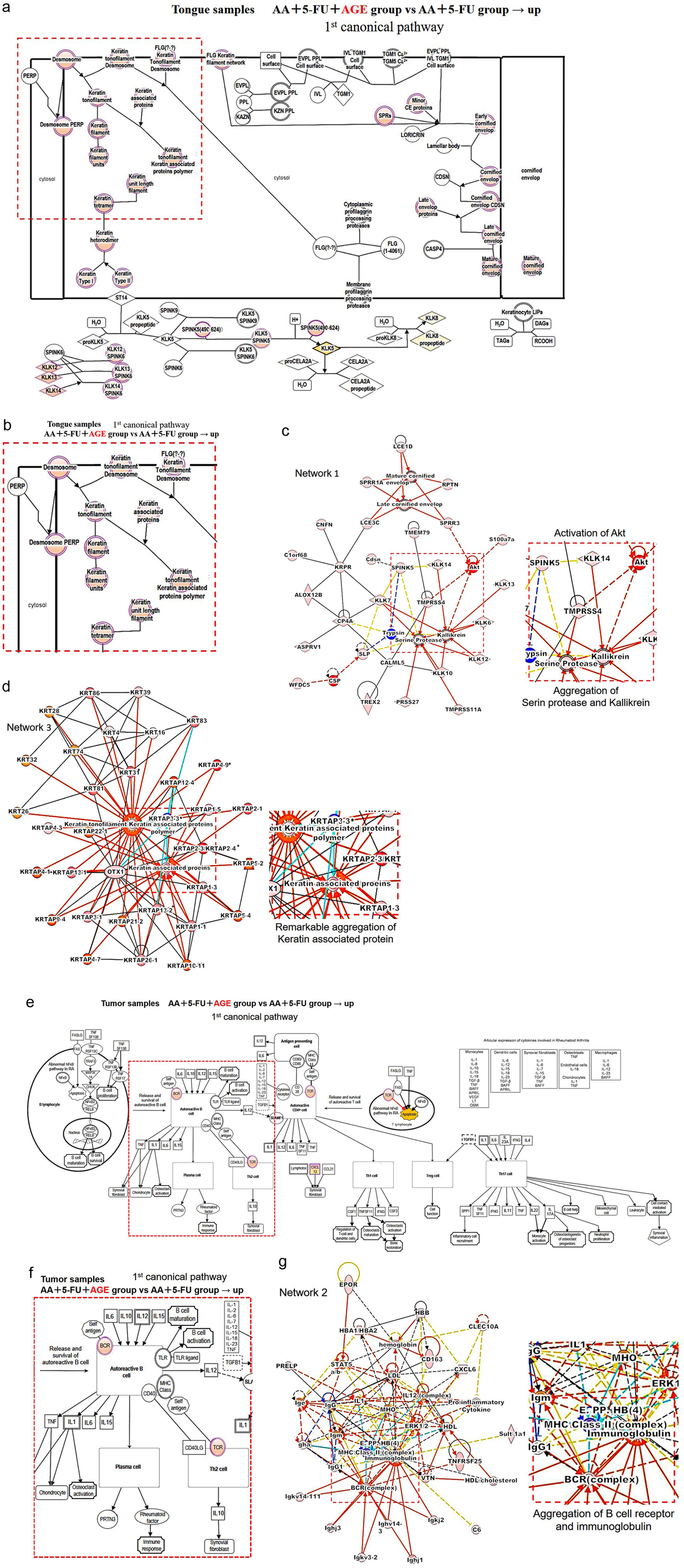 Click for large image | Figure 5. Mechanism of action of wound healing and antitumor effect of AGE. (a) The first canonical pathway for tongue samples. The genes marked in pink showed up-regulated expression between these two groups: AA + 5-FU + AGE vs. AA + 5-FU. An enlarged view of the marked area (by a red square) is shown in (b). (b) These “pink” genes were up-regulated in AA + 5-FU + AGE group, which are related to the keratinization process. (c) Network 1. The part inside this red square is enlarged on the right side. It shows the activation of Akt and the subsequent aggregation of serine protease and kallikrein in AA + 5-FU + AGE group. (d) Network 3. The enlarged view of the red square area is shown on the right side. There was a noticeable aggregation of keratin-associated proteins in AA + 5-FU + AGE group. (e) The first canonical pathway for tumor samples. Up-regulated gene expression between the two groups (AA + 5-FU and AA + 5-FU + AGE) is shown by pink color. An enlarged view of the marked area (by a red square) is shown in (f). (f) BCR and TCR were up-regulated in AA + 5-FU + AGE group. (g) Network 2. The red square area is enlarged on the right side. Aggregation of BCR (BCR complex) and immunoglobulin was observed in AA + 5-FU + AGE group. 5-FU: 5-fluorouracil; AA: acetic acid; AGE: aged garlic extract; BCR: B-cell receptor; TCR: T-cell receptor. |
Figures 5e shows the first canonical pathway for tumor samples. The marked (by a red square) part of Figure 5e is shown in Figure 5f (enlarged view). Between AA + 5-FU + AGE and AA + 5-FU groups, up-regulation of B-cell receptor (BCR) and T-cell receptor (TCR) was observed (Fig. 5f). Network analysis (network 2) also showed the aggregation of BCR complex and immunoglobulin in AA + 5-FU + AGE group (Fig. 5g).
Above findings suggest that AGE may heal ulcers in AA and 5-FU-induced oral mucositis by promoting normal cell differentiation processes including keratinization and re-epithelialization. Moreover, AGE may contribute to the antitumor effects of 5-FU in tumors through the activation of B cells.
| Discussion | ▴Top |
Oral mucositis caused by chemotherapy or chemoradiotherapy leads to basic physiological problems, such as eating and swallowing, increases the risk of malnutrition in cancer patients, and also strongly impairs quality of life. In our preliminary study, our aim was to clarify whether AGE can exert any healing effect on oral mucositis induced by AA and/or 5-FU in mouse model. The antitumor properties of AGE, and its influence on 5-FU antitumor activity were also examined in this study.
Our findings showed that the combination of 5-FU administration and AA application induced large mucositis areas in AA + 5-FU group which were healed by the administration of AGE in AA + 5-FU + AGE group (Fig. 3). In addition, the size and weight of the salivary glands was decreased in AA + 5-FU group compared to the control group, and administration of AGE could recover the gland size and weight in AA + 5-FU + AGE group (Fig. 4). These data suggested that AGE administration could reduce AA and 5-FU-induced oral mucositis in mice by reversing the negative effects of 5-FU, and by protecting the salivary glands against 5-FU-induced atrophic changes, and thereby restoring their functions. Acute mucositis can be caused by salivary hypofunction as well as insufficient antimicrobial factors and mucosal protectants (e.g. lactoferrin, defensins, glycoproteins, IgA, epidermal growth factor, mucins, etc.) [4, 36]. Lower saliva secretion volumes could be one of the reasons for the higher incidence of 5-FU-induced oral mucositis [4]. Taking the above facts into consideration, we can assume that the protective effects of AGE on the salivary glands against the 5-FU-induced atrophic changes might directly contribute to its healing effects on oral mucositis.
In this study, RNA sequencing and pathway analysis of mouse tumors and tongues were conducted to understand the molecular mechanism behind the healing effects and the antitumor properties of AGE. When the tongue samples of AA + 5-FU + AGE group were compared with AA + 5-FU + AGE group, IPA data showed activation of keratinization-related genes in AA + 5-FU + AGE group (Fig. 5b). Keratinocytes manufacture and store the keratin protein, and these proteins are responsible for the restoration of epidermis after injury through the epithelialization process, which is essential for covering the damaged epithelial surface [37]. A wound cannot be considered healed in the absence of keratinization and re-epithelialization [8]. Aggregation of keratin-associated proteins was also observed in IPA data (network 3, Fig. 5d). Moreover, pathway analysis also showed the activation of Akt kinases in AA + 5-FU + AGE group (Fig. 5c). Akt, a serine/threonine kinase, is crucial for maintaining epidermal homeostasis, as well as maintaining the function, differentiation, and survival of keratinocytes [39]. Akt kinases and phosphoinositide 3-kinases (PI3Ks)/Akt pathway modulate cellular survival and essential processes involved in acute wound healing [40]. Additionally, we observed the aggregation of serine protease and kallikrein in AA + 5-FU + AGE group (Fig. 5c). Serine proteases, including kallikrein-related peptidases are involved in wound healing and the repair of damaged epidermis [41, 42]. Therefore, our findings suggested that AGE may promote wound healing against AA and 5-FU-induced oral mucositis by promoting keratinization, re-epithelization, and normal cell differentiation process via the activation of Akt kinases, and accumulation of kallikrein and other serine proteases.
In this study, it was observed that AGE treatment somewhat enhanced the efficacy of 5-FU as tumor volume was significantly decreased in AA + 5-FU + AGE group compared to AA + 5-FU and AA groups (Fig. 2c). It is already documented that AGE has immunomodulatory effects [28, 30]. AGE contains pyruvates; namely, S-allylcysteine and S-allylmercaptocysteine which inhibit the growth and progression of cancer cells, and aid in cancer prevention [29-31, 43]. Our RNA sequencing and pathway analysis data showed the up-regulation of BCR and TCR as well as the aggregation of BCR complex and immunoglobulin in AA + 5-FU + AGE group (Fig. 5e-g). B cells are the main effector cells for several immunological pathways, which suppress tumor progression indirectly by secreting immunoglobulins and promoting the response of T cell, or directly by killing cancer cells [44]. Therefore, AGE may contribute to the antitumor effects of 5-FU in subcutaneous tumors through the activation of B cells. Our data indicate that AGE might improve the efficacy of chemotherapeutic drugs, such as 5-FU, or AGE itself may have anticancer effects.
Higher rate of cell proliferation and migration, angiogenesis, dermal matrix regeneration, and re-epithelization are prerequisites for wound healing [24, 45], and AGE promotes all of these processes [24, 26, 46]. However, cancer cells are also characterized by increasing and uncontrollable rate of cell proliferation, migration, invasion, and angiogenesis [47, 48]. Therefore, it is interesting that AGE could heal wounds in normal cells while showing antitumor effect in cancer cells (Figs. 2 and 3). We investigated this phenomenon in another study, and we observed that AGE affected non-cancerous cells (human keratinocyte cell and dermal fibroblast cell lines) differently than cancerous cells (OSCC and human gingival carcinoma cell lines) [46]. We reported that AGE promotes the growth and survival of normal cells through the induction of PKB or Akt family protein as well as brain-derived neurotrophic factor pathways, and inhibition of ferroptosis. On the other hand, AGE suppresses cancer cell proliferation by inhibiting cancer metastasis signaling processes and boosting phagocytic activity [46]. We concluded that AGE promotes the proliferation of normal cells but suppresses the growth of cancer cells through different cellular processes and signaling pathways [46]. However, detailed molecular mechanisms underlying the wound healing and antitumor properties of AGE are still largely unknown. We assume that as AGE contains multiple active ingredients with different functions, each ingredient may affect cells differently depending on the cell type, growth pattern or other characteristics (normal vs. cancer cells; wounded vs. functionally impaired cells). AGE may have many other unknown active ingredients and properties. Therefore, additional research is necessary to understand the role of AGE in normal and cancer cells, and to clarify the mechanisms under its wound healing and antitumor effects.
In this study, what was particularly interesting to us was the observation that tumor-bearing mice could recover from the 5-FU-induced weight loss after AGE treatment (Fig. 2b). Briefly, AGE not only had healing effects on mucositis but also improved mouse health. The anti-fatigue property of AGE has been known for many years and were explored in a number of studies [49, 50]. In addition, AGE treatment could heal the salivary glands even after severe damage and could recover their functions (Fig. 4a, b).
Our present study has certain limitations. The small sample size could result in low reproducibility of the data. Moreover, only female mice were used, which might introduce sex bias. Genomic analysis of tissue samples in a small cohort may also have the risk of generating unreliable data. However, this study aimed to obtain preliminary data on the healing effect of AGE on oral mucositis and to identify potential mechanisms of action of AGE. Therefore, four animals per group were used due to ethical considerations and to minimize animal use. Although a blinding method was not implemented in this study, the results were monitored and evaluated externally by a researcher during the course of the study, who carefully evaluated the data to ensure that all data were correct and adequate. We believe that our in vivo experimental protocol of mucositis is likely to produce results without high variability; nevertheless, future studies with a larger sample size will be necessary to validate the findings of our study. In our future studies, priori power calculation will be used to determine the appropriate sample size, and larger groups of both male and female mice will be used to improve data reliability and reproducibility.
About 30-40% of cancer patients receiving chemotherapy and almost 90% of head and neck cancer patients treated with chemoradiotherapy suffer from mucositis [51]. There are very few therapeutic options available for the treatment of mucositis, and the effectiveness of these therapies remains poor [14-17, 51]. In this scenario, a natural product like garlic, which has been traditionally used and appreciated for its wide range of potential medicinal benefits, including antibiotic, antibacterial, and antimycotic effects, hypocholesterolemic, antithrombotic, and antihypertensive activity, wound healing ability, and anticarcinogenic activity could be highly beneficial in the treatment of mucositis in cancer patients [19-31, 51, 52]. Several clinical studies have reported that AGE heals wounds by enhancing peripheral tissue perfusion and microcirculation in patients with arteriosclerosis and cardiovascular diseases [25, 26]; whereas other studies demonstrated its wound healing properties in pre-clinical settings [4, 24, 27]. The effectiveness of AGE against periodontal diseases is also studied clinically [32, 33]. AGE has high antioxidant and anti-inflammatory activities, which play major roles in wound healing [19-23, 28, 29]. Therefore, all of these beneficial properties of AGE could be responsible for its healing and protective effects against oral mucositis.
Our findings suggest that AGE could be effective against oral mucositis, and it may also enhance the antitumor activity of chemotherapeutic drugs in OSCC patients. Therefore, we believe that AGE treatment may have beneficial effects during cancer treatment, especially in patients receiving 5-FU-based therapies who are suffering from severe mucositis and/or a weakened immune system. We need clinical trials and extensive studies to fully understand the efficacy of AGE against oral mucositis and cancer. Our preliminary study could be useful for understanding the potential benefits of AGE treatment against mucositis, which might help in the development of new clinical trials and a novel AGE-based treatment regimen for OSCC patients in the future.
Acknowledgments
We would like to thank Associate Professor Cui Dan (Department of Pathology, Yamaguchi University Graduate School of Medicine) for her skilled technical support and helpful suggestions.
Financial Disclosure
This study was a joint research effort by the authors and Wakunaga Pharmaceutical Co., Ltd., Tokyo, Japan. This study was supported in part by a Grant-in-Aid for Scientific Research (grant no. 23K09354) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Wakunaga Pharmaceutical Co., Ltd.
Conflict of Interest
This study was partially supported by Wakunaga Pharmaceutical Co., Ltd., Tokyo, Japan. The company was not involved in the study design or data analysis, and will not bear the publication fees for this article.
Informed Consent
Not applicable.
Author Contributions
KH designed the study. KK, TF, and KH performed the experiments. KH, KW, KI, and YM analyzed and interpreted the data. KK, KH, and TF wrote and revised the manuscript. KM and YM contributed to the interpretation of data, revised the manuscript, and provided valuable suggestions throughout the study. All authors have read and approved the final version of the manuscript and are fully responsible for its content.
Data Availability
All data generated or analyzed during the present study are included in this published article. Whole transcriptome sequencing and IPA data are available with the permission of the authors upon request.
Abbreviations
AA: acetic acid; AGE: aged garlic extract; ANOVA: analysis of variance; BCR: B-cell receptor; 5-FU: 5-fluorouracil; H&E: hematoxylin and eosin; IgA: immunoglobulin A; IPA: ingenuity pathway analysis; OSCC: oral squamous cell carcinoma; PCR: polymerase chain reaction; PKB or Akt: protein kinase B; PI3Ks: phosphoinositide 3-kinases; SCCVII: mouse squamous cell carcinoma cells; SEM: standard error of the mean; TCR: T-cell receptor
| References | ▴Top |
- Duncan M, Grant G. Oral and intestinal mucositis - causes and possible treatments. Aliment Pharmacol Ther. 2003;18(9):853-874.
doi pubmed - Burdelya LG, Gleiberman AS, Toshkov I, Aygun-Sunar S, Bapardekar M, Manderscheid-Kern P, Bellnier D, et al. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):228-234.
doi pubmed - Sonis ST. Oral mucositis. Anticancer Drugs. 2011;22(7):607-612.
doi pubmed - McCarthy GM, Awde JD, Ghandi H, Vincent M, Kocha WI. Risk factors associated with mucositis in cancer patients receiving 5-fluorouracil. Oral Oncol. 1998;34(6):484-490.
doi pubmed - Miyano K, Ueno T, Yatsuoka W, Uezono Y. Treatment for cancer patients with oral mucositis: assessment based on the mucositis study group of the multinational association of supportive care in cancer in international society of oral oncology (MASCC/ISOO) in 2013 and proposal of possible novel treatment with a Japanese herbal medicine. Curr Pharm Des. 2016;22(15):2270-2278.
doi pubmed - Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(1):61-77.
doi pubmed - Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83(7):1433-1439.
doi pubmed - Kyllo RL, Anadkat MJ. Dermatologic adverse events to chemotherapeutic agents, part 1: cytotoxics, epidermal growth factor receptors, multikinase inhibitors, and proteasome inhibitors. Semin Cutan Med Surg. 2014;33(1):28-39.
doi pubmed - Shou J, Lieberman MD, Hofmann K, Leon P, Redmond HP, Davies H, Daly JM. Dietary manipulation of methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1991;15(3):307-312.
doi pubmed - Campos MI, Campos CN, Aarestrup FM, Aarestrup BJ. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol Clin Oncol. 2014;2(3):337-340.
doi pubmed - Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820-831.
doi pubmed - Peterson DE, Bensadoun RJ, Roila F, ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol. 2009;20(Suppl 4):174-177.
doi pubmed - Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, Sharp L. Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer. 2008;44(1):61-72.
doi pubmed - Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29(20):2815-2820.
doi pubmed - Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, Elting L, et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(Suppl 1):S1-21; quiz S22-24.
pubmed - Svanberg A, Ohrn K, Birgegard G. Oral cryotherapy reduces mucositis and improves nutrition - a randomised controlled trial. J Clin Nurs. 2010;19(15-16):2146-2151.
doi pubmed - Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck. 2004;26(1):77-84.
doi pubmed - Kodera Y, Kurita M, Nakamoto M, Matsutomo T. Chemistry of aged garlic: Diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions. Exp Ther Med. 2020;19(2):1574-1584.
doi pubmed - Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131(3s):1010S-1015S.
doi pubmed - Tsuneyoshi T. BACH1 mediates the antioxidant properties of aged garlic extract. Exp Ther Med. 2020;19(2):1500-1503.
doi pubmed - Morihara N, Hino A, Miki S, Takashima M, Suzuki JI. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol Nutr Food Res. 2017;61(10):1700308.
doi pubmed - Suzuki JI, Kodera Y, Miki S, Ushijima M, Takashima M, Matsutomo T, Morihara N. Anti-inflammatory action of cysteine derivative S-1-propenylcysteine by inducing MyD88 degradation. Sci Rep. 2018;8(1):14148.
doi pubmed - Miki S, Suzuki JI, Kunimura K, Morihara N. Mechanisms underlying the attenuation of chronic inflammatory diseases by aged garlic extract: Involvement of the activation of AMP-activated protein kinase. Exp Ther Med. 2020;19(2):1462-1467.
doi pubmed - Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim CW. Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol. 2009;32(3):191-203.
doi pubmed - Lindstedt S, Wlosinska M, Nilsson AC, Hlebowicz J, Fakhro M, Sheikh R. Successful improved peripheral tissue perfusion was seen in patients with atherosclerosis after 12 months of treatment with aged garlic extract. Int Wound J. 2021;18(5):681-691.
doi pubmed - Wlosinska M, Nilsson AC, Hlebowicz J, Malmsjo M, Fakhro M, Lindstedt S. Aged garlic extract preserves cutaneous microcirculation in patients with increased risk for cardiovascular diseases: A double-blinded placebo-controlled study. Int Wound J. 2019;16(6):1487-1493.
doi pubmed - Ramadan G, El-Beih NM, Ahmed RS. Aged garlic extract ameliorates immunotoxicity, hematotoxicity and impaired burn-healing in malathion- and carbaryl-treated male albino rats. Environ Toxicol. 2017;32(3):789-798.
doi pubmed - Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. J Nutr. 2001;131(3s):1075S-1079S.
doi pubmed - Suzuki J, Yamaguchi T, Matsutomo T, Amano H, Morihara N, Kodera Y. S-1-Propenylcysteine promotes the differentiation of B cells into IgA-producing cells by the induction of Erk1/2-dependent Xbp1 expression in Peyer's patches. Nutrition. 2016;32(7-8):884-889.
doi pubmed - Suzuki JI, Miki S, Ushijima M, Kodera Y. Regulation of immune response by S-1-propenylcysteine through autophagy-mediated protein degradation. Exp Ther Med. 2020;19(2):1570-1573.
doi pubmed - Lv Y, So KF, Wong NK, Xiao J. Anti-cancer activities of S-allylmercaptocysteine from aged garlic. Chin J Nat Med. 2019;17(1):43-49.
doi pubmed - Zini A, Mann J, Mazor S, Vered Y. The efficacy of aged garlic extract on gingivitis - a randomized clinical trial. J Clin Dent. 2018;29(2):52-56.
pubmed - Zini A, Mann J, Mazor S, Vered Y. Beneficial effect of aged garlic extract on periodontitis: a randomized controlled double-blind clinical study. J Clin Biochem Nutr. 2020;67(3):297-301.
doi pubmed - Snodgrass MJ, Burke JD, Meetz GD. Inhibitory effect of shark serum on the Lewis lung carcinoma. J Natl Cancer Inst. 1976;56(5):981-984.
doi pubmed - Saegusa Y, Ichikawa T, Iwai T, Goso Y, Ikezawa T, Nakano M, Shikama N, et al. Effects of acid antisecretory drugs on mucus barrier of the rat against 5-fluorouracil-induced gastrointestinal mucositis. Scand J Gastroenterol. 2008;43(5):531-537.
doi pubmed - Epstein JB, Tsang AH, Warkentin D, Ship JA. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(1):39-44.
doi pubmed - Konop M, Sulejczak D, Czuwara J, Kosson P, Misicka A, Lipkowski AW, Rudnicka L. The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017;25(1):62-74.
doi pubmed - Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445-464.
doi pubmed - Roy T, Boateng ST, Uddin MB, Banang-Mbeumi S, Yadav RK, Bock CR, Folahan JT, et al. The PI3K-Akt-mTOR and associated signaling pathways as molecular drivers of immune-mediated inflammatory skin diseases: update on therapeutic strategy using natural and synthetic compounds. Cells. 2023;12(12):1671.
doi pubmed - Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52-59.
doi pubmed - Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391(4):345-355.
doi pubmed - Kishibe M. Physiological and pathological roles of kallikrein-related peptidases in the epidermis. J Dermatol Sci. 2019;95(2):50-55.
doi pubmed - Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003;3(1):67-81.
doi pubmed - Tokunaga R, Naseem M, Lo JH, Battaglin F, Soni S, Puccini A, Berger MD, et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. 2019;73:10-19.
doi pubmed - Almadani YH, Vorstenbosch J, Davison PG, Murphy AM. Wound healing: a comprehensive review. Semin Plast Surg. 2021;35(3):141-144.
doi pubmed - Harada K, Ferdous T, Kawasaki K, Watanabe K, Mizukami Y. Effects of aged garlic extract may differ between normal cells and cancer cells. Biomed Rep. 2025;23(3):150.
doi pubmed - Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867.
doi pubmed - Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S, Nice EC, et al. Oral squamous cell carcinomas: state of the field and emerging directions. Int J Oral Sci. 2023;15(1):44.
doi pubmed - Wakunaga of America Co., Ltd. Aged Garlic Extract. Research Excerpts from Peer Reviewed Scientific Journals & Scientific Meetings. https://www.kyolic.ca/wp-content/uploads/2016/03/Aged-Garlic-Research-Excerpts.pdf. Accessed August 1, 2025.
- Morihara N, Nishihama T, Ushijima M, Ide N, Takeda H, Hayama M. Garlic as an anti-fatigue agent. Mol Nutr Food Res. 2007;51(11):1329-1334.
doi pubmed - Pulito C, Cristaudo A, Porta C, Zapperi S, Blandino G, Morrone A, Strano S. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res. 2020;39(1):210.
doi pubmed - Petrovska BB, Cekovska S. Extracts from the history and medical properties of garlic. Pharmacogn Rev. 2010;4(7):106-110.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.