| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 11, November 2025, pages 634-641
Loadless Tendon Explant Model Exhibits Spontaneous Degradative Phenotypes: An In Vitro Study of Human Tendons
Bing Shu Chena, Yi Jun Yuana, Yi Long Wanga, Si Yao Yangb, Wen Cui Lia, d, Jia Wei Guoa, c, d
aDepartment of Hand and Foot Surgery, Shenzhen Second People’s Hospital (The First Hospital Affiliated to Shenzhen University), Shenzhen 518028, China
bThe Medical Record Department, Shenzhen Second People’s Hospital (The First Hospital Affiliated to Shenzhen University), Shenzhen 518028, China
cGuangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen 518060, China
dCorresponding Authors: Wen Cui Li and Jia Wei Guo, Shenzhen Second People’s Hospital, Shenzhen 518028, Chinaand
Manuscript submitted August 2, 2025, accepted November 6, 2025, published online November 26, 2025
Short title: Loadless Human Tendon Degradation
doi: https://doi.org/10.14740/jocmr6343
| Abstract | ▴Top |
Background: Although the tendon explant model has been gradually recognized for simulating tendon characteristics in vitro, there is still no consensus on a standardized culture protocol. Recently, tendon explants have been applied to senescence-related research but whether their own changes affected the credibility of the conclusions was not confirmed. Therefore, this study aimed to systematically evaluate the reliability of the loadless tendon explant model by characterizing its dynamic macroscopic morphological and molecular biological changes during culture.
Methods: This study collected surgical samples from patients as loadless tendon explants for culture and conducted molecular biology and cytology tests once every 3 days within 15 days. Both their macroscopic and microscopic changes were recorded and analyzed, with each compared to the corresponding parameters at day 0.
Results: Loadless tendon explants spontaneously underwent progressive disintegration, leading to the collapse of the original dense structure. Consistently, the collagen fibers also gradually became loose and fractured, contributing to a significant decrease of collage density from day 9. In addition, the senescence and apoptosis markers P21 and Caspase 3 increased over time, and there were statistically significant differences starting from day 6, respectively. The main intrinsic component, Collagen I, and the main catabolic enzyme, metalloproteinase 13 (MMP 13), could hardly be detected since day 3. During the daily evaluations from day 0 to day 4, Collagen I gradually decreased and was barely detectable by day 3, whereas MMP 13 increased on day 1 followed by a rapid decline since day 2.
Conclusions: These results indicate that loadless tendon explants rapidly degrade and lose matrix integrity, with their spontaneous changes compromising the reliability of research conclusions related to senescence and apoptosis. Therefore, further optimization is still required before a well-established protocol is applied.
Keywords: Tendons; Collagen; Tissue transplantation; Senescence; Apoptosis
| Introduction | ▴Top |
Tendinopathy is a chronic tendon disease that has not been precisely defined, characterized by pain, limited function, and reduced exercise capacity. It is susceptible among athletes, manual laborers, the elderly, and obese individuals [1]. In the past three decades, the global incidence of tendinopathy has increased by 58% and it already accounts for 30-50% of all outpatients with musculoskeletal issues [2]. However, due to the unclear pathogenesis and complex pathological features of tendinopathy, there is no consensus on its treatment, which mainly includes pain management and physical therapy lasting for months or even years [3]. This imposes a huge mental and economic burden on patients and may potentially lead to heterogeneity in therapeutic efficacy. Therefore, exploring the mechanisms of tendinopathy is a prerequisite for an effective solution, and a reasonable experimental method is of first priority. Nevertheless, the dense structure of tendon tissue, its susceptibility to mechanical loading, and its requirement for a relatively hypoxic environment make it difficult to simulate the authentic state of tendon tissue in vitro [4-6]. Additionally, the spontaneous alterations of tendon tissue during culture may interfere with the results [7]. Thus, clarifying the biochemical and histological changes of tendon tissue during in vitro culture should precede the experiment, which is beneficial for ensuring the accuracy of conclusions.
The theoretical basis for in vitro tendon culture can be traced back to Bi et al, who successfully isolated and extracted tendon stem/progenitor cells from human and murine tendon tissues and confirmed their cellular stemness [8]. Currently, the tendon explant model based on intact tissue has been acknowledged by satisfied simulation of the in vivo situation of tendons, which helps to evaluate cell status and tissue characteristics [9, 10]. For example, the latest attractive research has applied it to the in vitro study of tendon aging [11]. However, the lack of a comprehensive evaluation of this model leads to uncertainties in the culture method and may affect the exactness of research. Previous studies merely focused on a few aspects such as collagen-related or proliferation gene expressions [12]. Therefore, a time-series analysis of the cellular and matrix changes of tendon explant model is necessary for relevant studies. Especially, research on human tendon explants will accelerate the possibility of clinical translation.
Thus, we intended to harvest the redundant parts of tendons discarded after autologous tendon reconstruction surgery from several patients for tendon explant culture. At different time points (day 0, day 3, day 6, day 9, day 12, day 15), cellular and molecular analyses of these samples were carried out by tissue staining and molecular biology techniques. Then the changes of collagen fibers, P21, Caspase 3, Collagen I, and matrix metalloproteinase 13 (MMP 13) over time were quantitatively evaluated. Collectively, this study assessed the feasibility of the existing tendon explant culture method, provided a theoretical basis for the improvement of those methods, and promoted the development of mechanism research on tendinopathy. The conclusion would benefit the basic research and clinical application in the use of human tendon explants for the tendinopathy treatment.
| Materials and Methods | ▴Top |
Ethical declaration
Our research was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. This study was approved by the Medical Ethics Committee of the Clinical Research Ethics Committee of Shenzhen Second People’s Hospital (2025-041-01PJ). All participants provided written consent for this study prior to the experiment.
Tendon explant management and culture
We enrolled volunteer patients who underwent autologous tendon reconstruction due to anterior cruciate ligament rupture. The specific inclusion and exclusion criteria were described.
Inclusion criteria
1) Tendon samples are obtained from the semitendinosus muscle. 2) Aged 18 - 60 to exclude physiological degeneration-related tendon quality differences. 3) No obvious macroscopic damage or degeneration of the semitendinosus tendon (e.g., no tear, calcification, fibrosis, abnormal color). 4) Preoperative magnetic resonance imaging (MRI) confirms normal structure of the semitendinosus tendon without organic lesions.
Exclusion criteria
1) History of systemic diseases: obesity (body mass index (BMI) ≥ 30 kg/m2), type 2 diabetes, hyperlipidemia, hypothyroidism, rheumatoid arthritis, hyperthyroidism, hyperparathyroidism, chronic renal failure, systemic lupus erythematosus, severe liver insufficiency, ankylosing spondylitis, and malignant tumors. 2) History of tendon-related diseases (e.g., tendinitis, tendon tear, calcification, and enthesopathy). 3) Trauma or surgical history at the sample site within the past 6 months. 4) History of infection at the sample site or systemic infection. 5) Long-term (past 3 months) use of glucocorticoids, immunosuppressants, chemotherapeutic drugs, etc. 6) Long-term smoking (≥ 10 cigarettes/day for ≥ 5 years) or excessive alcohol consumption (≥ 25 g alcohol/day).
Five male patients aged 24 - 46 participated. The discarded tendons during the surgery were collected for culture, histological staining, and molecular biology experiments. They were immersed in low-glucose Dulbecco’s modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin [11]. Each of them was evenly and sterilely divided into 12 parts. Then they were randomly distributed on a plate, with 2 mL of culture medium added. Subsequently, they were cultured at 37 °C, 5%CO2, and without mechanical load. Medium was replaced every 24 h. We also harvested tendons from another three male patients aged 30 - 36 to perform subsequent protein quantitation every day. The collection and culture methods were the same as the previous ones.
Morphological observation
We performed sampling and recording on day 0/3/6/9/12/15. At each time point, two samples were collected from each patient. Each sample was washed three times with double-distilled water to remove surface impurities. Photographs were taken at different time points.
Histological staining
To investigate the collagen content, cellular senescence, and apoptosis of tendon explants, five independent samples at each time point were immersed in 4% paraformaldehyde (PFA) (Servicebio, G1101, Wuhan, China) at room temperature for more than 48 h. Then, 5 µm paraffin slices were prepared.
We used the kit (Solarbio, G1340, Beijing, China) to perform the Masson staining. The slices were stained with Ponceau-Fuchsin for 5 min, then washed with 1% phosphomolybdic acid for 1 min. Subsequently, they were stained with Aniline Blue for 2 min and washed with 1% ethanoic acid for 1 min.
For the immunohistochemical staining, the slices were heated in sodium citrate buffer (pH = 6.0) at 95 °C for 20 min to complete antigen retrieval. After cooling to room temperature, they were incubated with 3% H2O2 for 10 min, followed by incubation with phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) for 30 min. Subsequently, they were incubated overnight at 4 °C with diluted P21 polyclonal antibody (Proteintech, 10355-1-AP, 1:200, Wuhan, China) or Caspase 3 polyclonal antibody (Proteintech, 19677-1-AP, 1:200, Wuhan, China). After washing with PBS, they were incubated with Goat anti-rabbit IgG H&L (HRP) secondary antibody (Abcam, ab6721, 1:1,000, Cambridge, UK) at room temperature for 1h, and then developed with 3,3-diaminobenzidine (DAB) (Beyotime, P0203, Shanghai, China).
All slices were routinely dehydrated, cleared, and mounted for imaging. The percentage of collagen fiber area and positive cells were analyzed using ImageJ software. 1) Collagen fiber density. RGB images of sections were opened, and the blue-stained collagen fibers were selectively segmented via the “Threshold” function by adjusting blue channel parameters. After confirming the selected collagen regions, the “Set Measurements” function was activated with the “Area” and “Limit to threshold” options enabled. Collagen fiber area was measured by pressing the “M” key, and the total image area was calculated as the product of image width and height in pixels. The collagen fiber density was finally computed as (collagen fiber area/total image area) × 100%. 2) Positive cell rate. RGB images of sections were imported, and DAB-positive cells (brown signal) were segmented via the “Threshold” function by adjusting red/green channel parameters to exclude background. Positive cells were automatically counted with “Analyze Particles” (size and circularity optimized by target cell morphology) after enabling “Cell Count” and “Limit to threshold” in “Set Measurements”. Total nucleated cells were quantified by selecting hematoxylin and eosin (H&E)-stained nuclei (blue) via threshold adjustment, and the positive cell rate was calculated as (number of DAB-positive cells/total H&E-stained nucleated cells) × 100%.
Western blot
To investigate the protein expression of anabolism and catabolism in tendon explants, five independent samples at each time point were lysed using radioimmunoprecipitation assay (RIPA, Beyotime, P0013B, Shanghai, China). The protein concentration of the supernatant was determined by the bicinchoninic acid (BCA) method. Subsequently, the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking the membrane with 5% skim milk, it was incubated overnight at 4 °C with Rabbit anti-Collagen I polyclonal antibody (Bioss, bs-0578R, 1:1,000, Beijing, China), or Rabbit anti-MMP 13 polyclonal antibody (Bioss, bs-10581R, 1:1,000, Beijing, China), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Proteintech, 60004-1-Ig, 1:50,000, Wuhan, China). Subsequently, the membranes were incubated with Goat anti-rabbit IgG H&L (horseradish peroxidase (HRP)) (Abcam, ab6721, 1:10,000, Cambridge, UK) or Rabbit anti-mouse IgG H&L (HRP) (Abcam, ab6728, 1:10,000, Cambridge, UK) secondary antibody at 37 °C for 2 h, and then developed with DAB (Beyotime, P0203, Shanghai, China). The other three independent samples were managed as the same.
Statistical analysis
SPSS 13.0 software was adopted. All data were presented as mean ± standard deviation (SD). Comparison was conducted by using repeated measures one-way analysis of variance (ANOVA), compared to day 0. P < 0.05 was considered statistically significant.
| Results | ▴Top |
Loadless tendon explants exhibited spontaneous collapse and disintegration
Firstly, we collected the redundant tendon tissues from five male patients aged 24 - 46 who underwent anterior cruciate ligament reconstruction on the same day (Fig. 1a). Regarding that the potential influences of obesity, inflammation, nutrition, and metabolites, we analyzed their baseline data. The results showed that their BMI, white blood cell count, platelet count, hemoglobin level, albumin level, creatinine level, and uric acid level were basically within the normal range (Table 1). Each tendon tissue was immediately divided into 12 equal parts, and the loadless tendon explants were cultured in vitro as previously reported (Fig. 1b). To dynamically evaluate the macroscopic morphological changes of the tendon explants, we obtained images every 3 days within 15 days of the culture, so as to compare the macroscopic changes. The results showed that the tendon explants from all five patients exhibited spontaneous disintegration: as the culture time prolonged, the volume of the tendons gradually decreased, the surface became loose, and the tendon fiber bundles separated (Fig. 2).
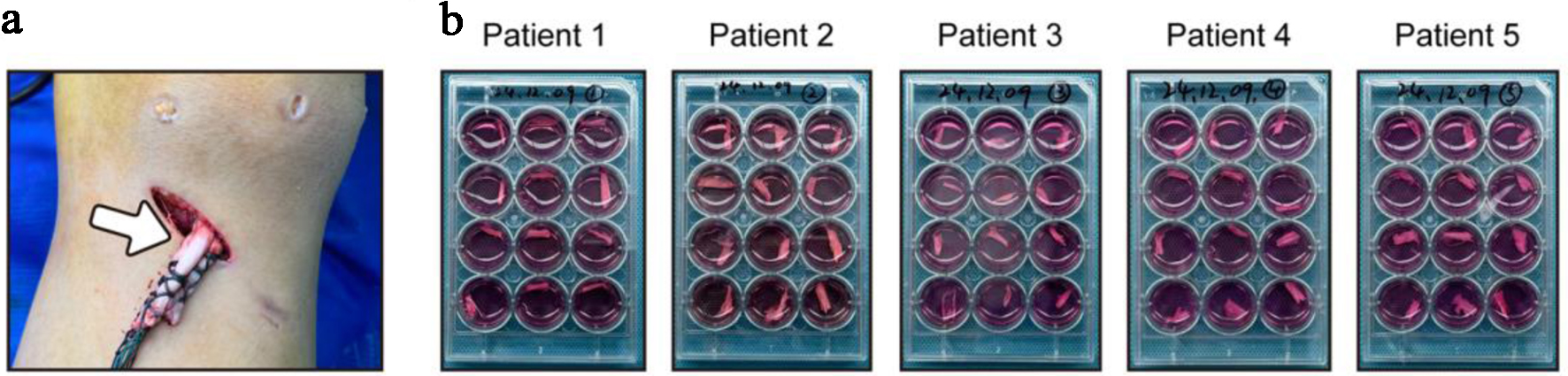 Click for large image | Figure 1. Redundant tendon tissues were intraoperatively harvested and cultured without load in vitro. (a) Redundant tendon tissues were intraoperatively harvested from patients after anterior cruciate ligament reconstruction. (b) Tendon tissues were divided into 12 equal parts and cultured without load. |
 Click to view | Table 1. Baseline Data of the Five Patients |
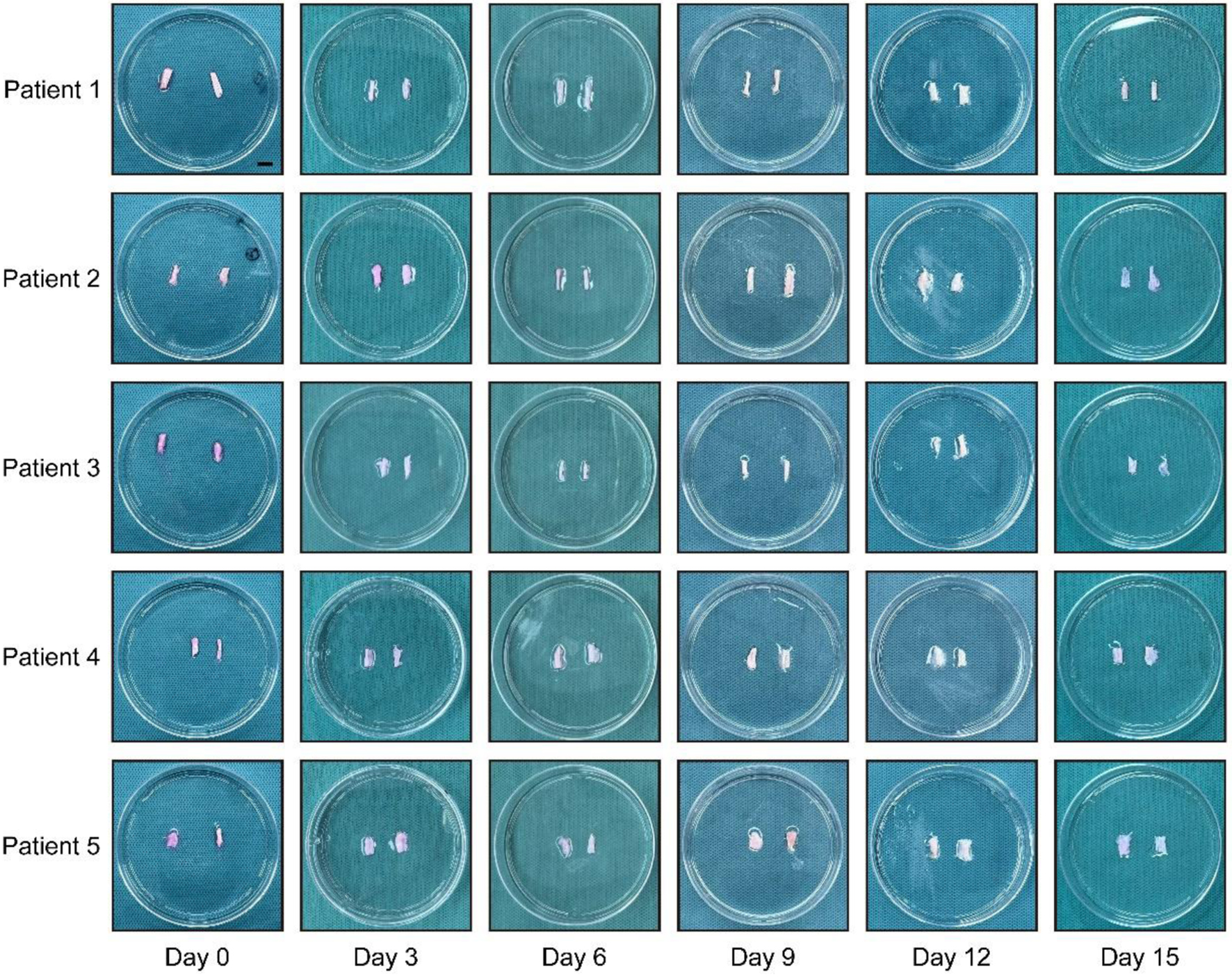 Click for large image | Figure 2. Loadless tendon explants underwent spontaneous disintegration. Images showing morphological changes of tendon explants on day 0, 3, 6, 9, 12, and 15 from the five patients, respectively. Scale bar = 1 cm. |
In order to identify those changes from microscopic perspective, we performed Masson staining of the samples at each time point. The results showed that the internal collagen network collapsed over time, accompanied by density decrease and rupture of the local collagen fibers. Specifically, the collagen arrangement in the tendons on day 0 presented a dense parallel bundle structure, which was significantly disintegrated by day 9 (Fig. 3a). The quantitative analysis showed that the collagen density of the tendon fibers gradually decreased. Compared to day 0, it decreased by approximately 10% on day 9, with statistical significance. Subsequently, the collagen density decreased by approximately 20% on day 15 (Fig. 3d).
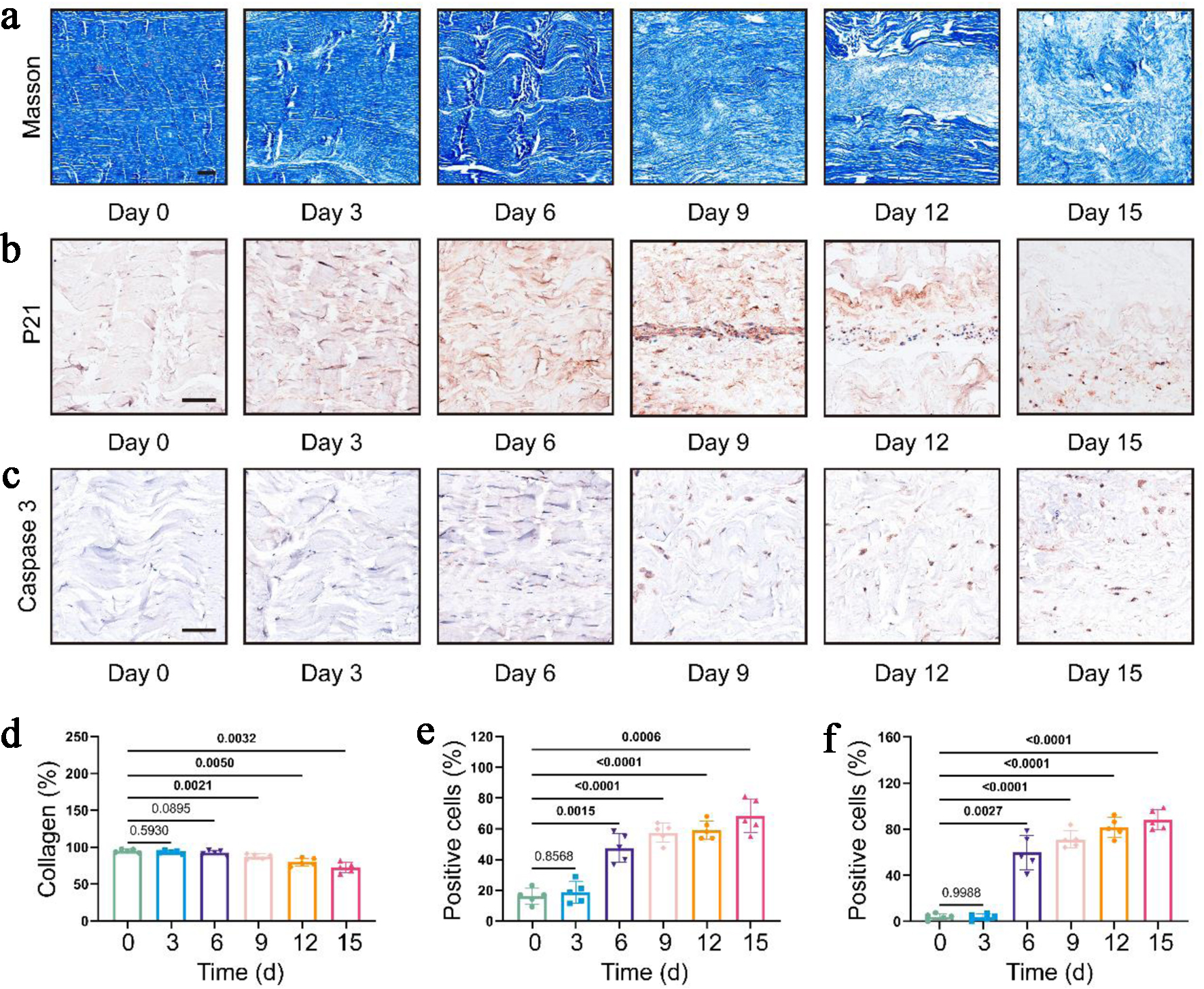 Click for large image | Figure 3. Cell and matrix in loadless tendon explants demonstrated degradative phenotypes. (a) Representative images of Masson staining at different time points. Scale bar = 100 µm. (b, c) Representative images of immunohistochemical staining of P21 and Caspase 3 at different time points, respectively. Scale bar = 100 µm. (d-f) Quantitative analysis of collagen fiber density (d), P21-positive cell rate (e), and Caspase 3-positive cell rate (f), respectively. Data were presented as mean ± SD (n = 5). Repeated measures one-way ANOVA (compared to day 0) were adopted for statistical analysis. The P values have been annotated accordingly, with values indicating statistical significance bolded. ANOVA: analysis of variance; SD: standard deviation. |
Loadless tendon explants displayed cellular degradative phenotypes
Next, to evaluate the cell status of the tendon explants at various time points, we performed immunohistochemical staining for the senescence marker P21 and the apoptosis marker Caspase 3. The results showed that the number of positive cells for both markers increased significantly starting from day 6 (Fig. 3b, c). Quantitative analysis revealed that compared with day 0, the positive cell rate of P21 increased significantly from 15% to approximately 50%, and it was approximately 70% on day 15 (Fig. 3e). Similarly, the positive cell rate of Caspase 3 increased significantly from 5% to approximately 60%, and it was approximately 90% on day 15 (Fig. 3f).
Collagen I in loadless tendon explants decreased rapidly
Collagen I is the main structural component of the extracellular matrix of tendons, which significantly affects the stability of tendon tissues [13]. Its content usually accounts for more than 90% of the tissue [14]. To evaluate the compositional changes of tendon explants at various time points, we performed Western blot for it. The results showed that on day 0, Collagen I was highly expressed in the tendon tissues. However, its level decreased significantly on day 3 and could barely be detected at following time points (Fig. 4a). In addition, normal tendon tissues contain some MMP 13 to maintain tissue renewal, while the content increases under pathological conditions [15]. The results showed low-level MMP 13 expression on day 0 and day 3, and it could no longer be detected from day 6 to day 15 (Fig. 4a). To elucidate their change pattern within the first few days, we harvested tendons from another three patients to carry out analysis daily. Consistently, Collagen I decreased gradually, especially from day 2 to day 3. Meanwhile, MMP 13 increased within 2 days but decreased dramatically since day 2 (Fig. 4b).
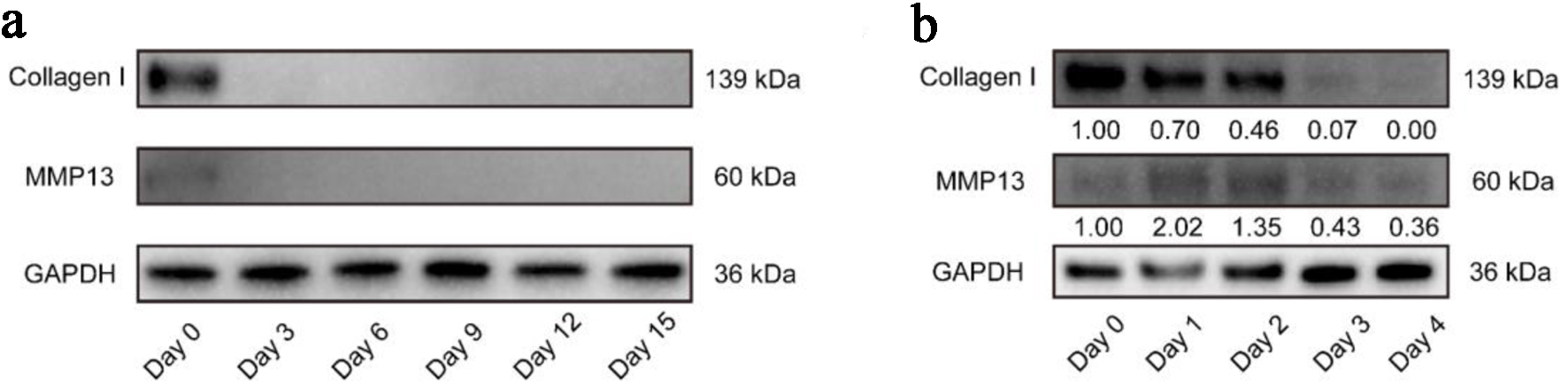 Click for large image | Figure 4. Western blot showing decreasing trend of Collagen I and MMP 13 expression in tendon explants over time. Representative images showing Collagen I and MMP 13 expressions from day 0 to day 15 (a) or from day 0 to day 4 (b). MMP: metalloproteinase 13. |
| Discussion | ▴Top |
To elaborate the pathogenesis of tendinopathy, previous studies have proposed that the method of tendon explants can help evaluate the cell state and tissue characteristics [16]. However, this method has not yet reached a consensus, and there is a lack of systematic and dynamic evaluation during the culture. Herein, we collected human tendons and analyzed them at cellular and molecular levels at different time points. The results revealed that loadless human tendon explants underwent structural disintegration and cellular degradative phenotypes, indicating that the method of tendon explants still requires reasonable optimization and improvement. Otherwise, their spontaneous alterations will ultimately affect the credibility of related studies.
Compared with previous studies, our study included the macroscopic morphological evaluation of loadless tendon explants and found the progressive tissue disintegration. This was consistent with the results reported by Wang et al. That is, after culture with load deprivation, tendons exhibit degenerative changes and weakened mechanical properties. Moreover, they found that regaining mechanical stimulation could rescue the pathological changes and mechanical properties of the tendons [17]. Thus, mechanical stimulation was crucial for maintaining the stability of tendon tissue.
The absence of the original mechanical microenvironment can simultaneously trigger cell cycle arrest and cell state alterations [18]. In our experiment, the cell senescence and apoptosis markers P21 and Caspase 3 continuously accumulated. This is in line with Stowe et al, whose results showed P21 expression increased during the period from day 0 to day 7 during tendon explant culture [11]. Similarly, Andarawis-Puri et al, who used Caspase 3 staining to quantify the positive cells in patellar tendon, found that the number on day 7 was higher than that on day 3 [19]. Interestingly, we observed transiently active proliferation in local areas during the period from day 6 to day 12. We speculated that tendon disintegration promoted the contact and substance exchange between cells and the culture medium. It may also be related to the activation of the compensatory metabolic pathways of cells [20, 21]. In addition, the increasing ratio of senescent or apoptotic cells slowed in the late stage. Through Masson staining, we suspected that this could attribute to the impairment of structural support, which impeded the cell to adhere to the matrix. This also suggested that the integrity of the tendon tissue structure was a prerequisite for cell survival. Regarding this conjecture, analyzing the cells in the supernatant may identify the responsible factors.
Collagen I is the main structural component of the tendon tissue and a decisive component of its function [22, 23]. In this experiment, Collagen I in loadless tendon explants was highly expressed on day 0 but was nearly undetectable from day 3. This is completely different from the situations verified in previous experiments, in which the expression of Collagen I increased [24]. At the same time, MMP 13 has a low-level expression to help maintain the normal renewal of tendon tissue [15]. Consistent with this, we detected its low-level expression on day 0, but it could hardly be detected from day 3. Despite the insufficiency of the existing evidence for attribution, all of them implied that significant catabolism occurred in loadless tendon explants from day 0 to day 3. These results indicated that the method in this study could not simulate the physiological state of tendon tissue in vivo due to the spontaneous changes throughout the culture.
We acknowledge the limitations of this study. The narrow sample may introduce biological bias and restrict generalizability to other demographics (e.g., older individuals, females, those with tendinopathy). Besides, our observational study has not yet proposed specific methodological optimization strategies, and targeted interventional investigations are warranted in subsequent research to address this gap.
Collectively, this experiment has elucidated that this loadless tendon explant demonstrates spontaneously catabolic state at both biochemical and cellular levels. We believe that this method requires optimization in multiple aspects. Firstly, the enlarged sample size and age stratification should be considered. Secondly, more intensive sampling points can be set up in the initial stage of culture, and highly sensitive detection techniques can be applied to clarify the changes of Collagen I and MMP 13. Thirdly, multiple markers related to stress, proliferation, etc., can be evaluated. Fourthly, mechanical load, oxygen concentration, and medium components can be regarded as control variables to mimic in vivo environment of tendons to the greatest extent. Through those improvements, tendon explants can be more promising in exploring the tendinopathy mechanism.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP007), Shenzhen Key Medical Discipline Construction Fund (No. SZXK025), Sanming Project of Medicine in Shenzhen (No. SZSM202311008), and Shenzhen Science and Technology Program (JCYJ20230807115121043). Project funded by China Postdoctoral Science Foundation (2023M742413).
Conflict of Interest
All contributing authors declare no conflict of interest.
Informed Consent
All tendon samples utilized in this study were obtained from the Biobank of Shenzhen Second People’s Hospital, where donors had previously provided broad written informed consent allowing the use of their samples and associated anonymized data for future medical research. The current study protocol falls within the scope of this original consent. Informed consent was obtained from the subjects involved in the study.
Author Contributions
JWG and BSC took the lead in writing the manuscript. JWG and YJY designed experiments and analyzed data. BSC and YLW was responsible for collecting tendon specimens and experimental data. SYY collected data. JWG and WCL conceived the study, secured funding, and supervised overall direction. All authors contributed critical feedback that shaped the study and manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Andarawis-Puri N, Flatow EL. Promoting effective tendon healing and remodeling. J Orthop Res. 2018;36(12):3115-3124.
doi pubmed - Jin Z, Wang D, Zhang H, Liang J, Feng X, Zhao J, Sun L. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis. 2020;79(8):1014-1022.
doi pubmed - Verges J, Martinez N, Pascual A, Bibas M, Santina M, Rodas G. Psychosocial and individual factors affecting Quality of Life (QoL) in patients suffering from Achilles tendinopathy: a systematic review. BMC Musculoskelet Disord. 2022;23(1):1114.
doi pubmed - Tarantino D, Mottola R, Resta G, Gnasso R, Palermi S, Corrado B, Sirico F, et al. Achilles tendinopathy pathogenesis and management: a narrative review. Int J Environ Res Public Health. 2023;20(17):6681.
doi pubmed - Elliot D. Staged tendon grafts and soft tissue coverage. Indian J Plast Surg. 2011;44(2):327-336.
doi pubmed - Mzeihem M, El Bachour J, Hemdanieh M, El Baba B, Tamim H, Nassereddine M. Achilles tendon rupture primary repair technique: A comparative retrospective study between graft versus no-graft. J Clin Orthop Trauma. 2024;51:102417.
doi pubmed - Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99(3):203-222.
doi pubmed - Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219-1227.
doi pubmed - Dyment NA, Barrett JG, Awad HA, Bautista CA, Banes AJ, Butler DL. A brief history of tendon and ligament bioreactors: Impact and future prospects. J Orthop Res. 2020;38(11):2318-2330.
doi pubmed - Bramson MTK, Van Houten SK, Corr DT. Mechanobiology in tendon, ligament, and skeletal muscle tissue engineering. J Biomech Eng. 2021;143(7):070801.
doi pubmed - Stowe EJ, Keller MR, Connizzo BK. Cellular senescence impairs tendon extracellular matrix remodeling in response to mechanical unloading. Aging Cell. 2024;23(11):e14278.
doi pubmed - Troop LD, Puetzer JL. Intermittent cyclic stretch of engineered ligaments drives hierarchical collagen fiber maturation in a dose- and organizational-dependent manner. Acta Biomater. 2024;185:296-311.
doi pubmed - Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10(6):312-320.
doi pubmed - Franchi M, Trire A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007;7:404-420.
doi pubmed - Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K, Shender MA. Loss of homeostatic strain alters mechanostat "set point" of tendon cells in vitro. Clin Orthop Relat Res. 2008;466(7):1583-1591.
doi pubmed - Wunderli SL, Blache U, Snedeker JG. Tendon explant models for physiologically relevant invitro study of tissue biology - a perspective. Connect Tissue Res. 2020;61(3-4):262-277.
doi pubmed - Wang T, Lin Z, Ni M, Thien C, Day RE, Gardiner B, Rubenson J, et al. Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J Orthop Res. 2015;33(12):1888-1896.
doi pubmed - Aggouras AN, Stowe EJ, Mlawer SJ, Connizzo BK. Aged tendons exhibit altered mechanisms of strain-dependent extracellular matrix remodeling. J Biomech Eng. 2024;146(7):071009.
doi pubmed - Andarawis-Puri N, Philip A, Laudier D, Schaffler MB, Flatow EL. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. J Orthop Res. 2014;32(9):1097-1103.
doi pubmed - Paredes J, Marvin JC, Vaughn B, Andarawis-Puri N. Innate tissue properties drive improved tendon healing in MRL/MpJ and harness cues that enhance behavior of canonical healing cells. FASEB J. 2020;34(6):8341-8356.
doi pubmed - Kallenbach JG, Freeberg MAT, Abplanalp D, Alenchery RG, Ajalik RE, Muscat S, Myers JA, et al. Altered TGFB1 regulated pathways promote accelerated tendon healing in the superhealer MRL/MpJ mouse. Sci Rep. 2022;12(1):3026.
doi pubmed - Engebretson B, Mussett ZR, Sikavitsas VI. Tenocytic extract and mechanical stimulation in a tissue-engineered tendon construct increases cellular proliferation and ECM deposition. Biotechnol J. 2017;12(3):1600595.
doi pubmed - Taye N, Karoulias SZ, Hubmacher D. The "other" 15-40%: The role of non-collagenous extracellular matrix proteins and minor collagens in tendon. J Orthop Res. 2020;38(1):23-35.
doi pubmed - Zhang J, Wang JH. The effects of mechanical loading on tendons—an in vivo and in vitro model study. PLoS One. 2013;8(8):e71740.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.