| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 11, November 2025, pages 642-652
The Risk of Tuberculosis in Chronic Obstructive Pulmonary Disease Across Different Comorbidities
Kuang-Ming Liaoa, Lu-Ting Kuob, Hsueh-Yi Luc, d
aDepartment of Internal Medicine, Chi Mei Medical Center, Chiali, Taiwan, Republic of China
bDivision of Neurosurgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan, Republic of China
cDepartment of Industrial Engineering and Management, National Yunlin University of Science and Technology, Yun-Lin, Taiwan, Republic of China
dCorresponding Author: Hsueh-Yi Lu, Department of Industrial Engineering and Management, National Yunlin University of Science and Technology, Yun-Lin, Taiwan, Republic of China
Manuscript submitted August 3, 2025, accepted November 4, 2025, published online November 26, 2025
Short title: Risk of TB in COPD Across Different Comorbidities
doi: https://doi.org/10.14740/jocmr6345
| Abstract | ▴Top |
Background: Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder with significant global morbidity and mortality. COPD is increasingly recognized as a systemic inflammatory condition that predisposes patients to multiple comorbidities, including tuberculosis (TB). There are limited data on how comorbidities in COPD influence the development of TB.
Methods: We conducted a nationwide, retrospective cohort study using data from Taiwan’s National Health Insurance Research Database (NHIRD) between 2011 and 2021. Patients aged ≥ 40 years with a diagnosis of COPD, confirmed by ≥ 3 outpatient visits or ≥ 1 hospitalization, were included. Individuals with prior TB were excluded. Non-COPD controls were matched 1:1 using propensity score matching for demographics and comorbidities. The primary outcome was incident TB (ICD-9-CM 010-018). Cox proportional hazards models were used to estimate adjusted hazard ratios (aHRs) for TB, accounting for a variety of comorbidities.
Results: A total of 117,989 COPD patients and an equal number of matched controls were analyzed. During follow-up, TB incidence was significantly higher in the COPD group (3.20 vs. 1.45 per 10,000 person-years). COPD was associated with a 74% increased risk of TB (aHR 1.74; 95% confidence interval (CI): 1.42 - 2.14; P < 0.001). Stratified analysis demonstrated that TB risk rose progressively with age and was markedly amplified by comorbidities. Notably, pneumoconiosis conferred a nearly sevenfold higher TB risk among COPD patients (aHR 6.94; 95% CI: 2.72 - 17.71; P < 0.001), followed by lung cancer (aHR 1.81; 95% CI: 1.07 - 3.05; P < 0.027) and rheumatoid arthritis (aHR 2.05; 95% CI: 1.01 - 4.12; P < 0.046).
Conclusion: After adjustment for available covariates, COPD was associated with an increased risk of TB; however, residual confounding due to factors such as corticosteroid use, immunosuppressive therapy, and smoking cannot be excluded.
Keywords: Chronic obstructive pulmonary disease; Comorbidity; Pneumoconiosis; Tuberculosis
| Introduction | ▴Top |
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder characterized by persistent airflow limitation and chronic inflammation. It manifests as chronic cough, sputum production, and exertional dyspnea, affecting a large global population and representing a leading cause of morbidity and mortality. Beyond the lungs, COPD is increasingly recognized as a systemic inflammatory condition with extra-pulmonary effects on the cardiovascular system, skeletal muscles, bones, and kidneys. Comorbidities are highly prevalent in COPD, with pulmonary tuberculosis (TB) being an important yet underrecognized one. Increasing evidence suggests that COPD independently raises the risk of active TB [1]. This association is likely multifactorial, reflecting shared risk factors such as smoking [2, 3], poor nutrition, low socioeconomic status [4, 5], and impaired immune responses [6], as well as overlapping structural lung damage. Epidemiological studies demonstrate considerable overlap between COPD and TB. Approximately one-third of COPD patients have a prior history of pulmonary TB [7], supporting the notion that TB may contribute to COPD pathogenesis. Conversely, a meta-analysis of 23 studies reported that individuals with prior TB had 2.59-fold higher odds of developing COPD [8], suggesting long-term structural and inflammatory damage. A large nationwide, multicenter, prospective cohort study is ongoing in China to evaluate the natural history and clinical burden of TB-associated COPD [9], particularly in regions where both diseases are highly prevalent. Compared to Western countries, Asian countries continue to experience a significantly higher prevalence and incidence of pulmonary TB [10], likely due to a combination of socioeconomic, environmental, and healthcare-related factors [11]. TB is a clinically important consideration in patients with COPD. Overlapping respiratory symptoms between COPD and TB can complicate timely diagnosis, potentially delaying appropriate treatment and worsening patient outcomes. Therefore, recognizing and accounting for TB risk is essential to ensure the safe and effective management of COPD patients, including appropriate screening, preventive measures, and careful monitoring during therapy. Despite this, there remains a paucity of research specifically addressing the impact of COPD comorbidities on pulmonary TB. There is a need to better understand how these comorbid conditions interact and influence pulmonary TB. Therefore, the primary aim of our study was to investigate the association between various comorbidities and the development of pulmonary TB among patients with COPD in an Asian population. By identifying risk factors and patterns of disease overlap, we hope to contribute to more effective prevention, early diagnosis, and targeted treatment strategies for this vulnerable group of patients.
| Materials and Methods | ▴Top |
Ethics statement
The study was approved by the Institutional Review Board of Chi Mei Medical Center, Taiwan (IRB No. 11303-J01), with informed consent waived and all personal data anonymized by the Data Science Center, Ministry of Health and Welfare.
Data sources
The Ministry of Health and Welfare in Taiwan has implemented a nationwide health system, the National Health Insurance (NHI) program, which provides near-universal coverage - encompassing approximately 99% of the population and contracting with over 97% of healthcare providers across the country. To facilitate epidemiological and health services research, the Ministry of Health and Welfare established the National Health Insurance Research Database (NHIRD), administered by the Health and Welfare Data Science Center, with data available upon approved application. The NHIRD represents one of the largest administrative healthcare databases globally, containing comprehensive records of all inpatient and outpatient encounters reimbursed by the NHI. These data include demographic profiles, diagnostic and procedural codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), prescription details, and medical expenditure information. For the present study, a longitudinal dataset was utilized comprising a representative cohort of two million individuals randomly selected from the insured population, with retrospective follow-up from 2011 through 2021. All data analyses were conducted within the secure computing environment of the Data Science Center, adhering to rigorous deidentification protocols. Personal identifiers were removed prior to analysis, and the cohort was validated to ensure no statistically significant differences in age, sex, or healthcare cost distributions compared to the general NHI population.
Patients
Individuals with COPD were identified based on diagnoses coded using the ICD-9-CM codes 490-492 and 496, recorded between January 1, 2011, and December 31, 2021. To enhance diagnostic validity, eligible subjects were required to have either a minimum of three outpatient visits or at least one hospitalization in which COPD was listed as the primary diagnosis within the NHIRD. The date of the third outpatient visit or the first qualifying inpatient admission - whichever occurred earlier - was defined as the index date for subsequent TB risk assessment. Following the application of exclusion criteria, which included individuals younger than 40 years of age, those with a history of TB prior to the index date, and records with incomplete data, a total of 122,285 COPD patients were retained for further analysis (Fig. 1).
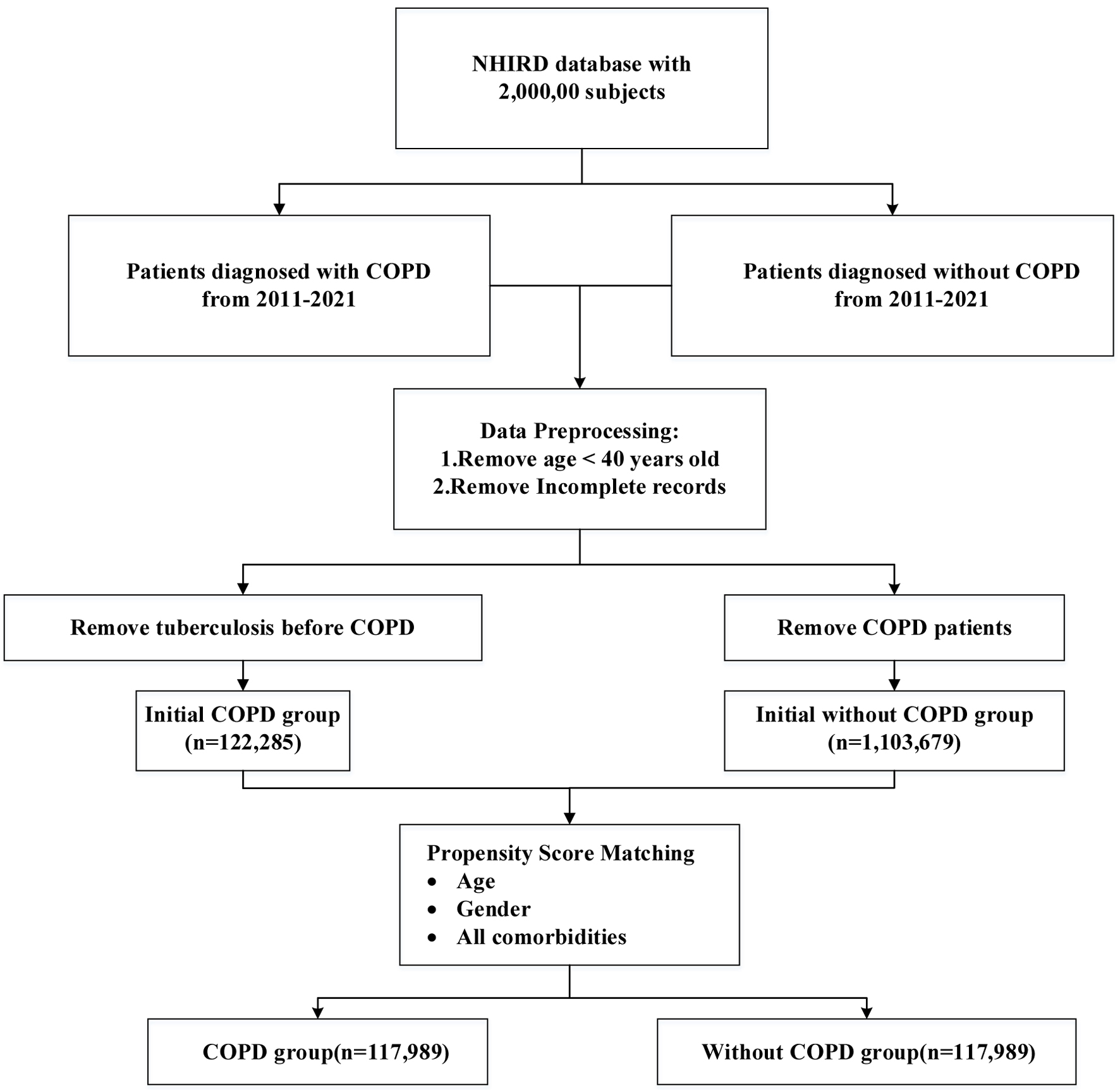 Click for large image | Figure 1. Flowchart of subject enrollment. |
Propensity score matching
The control group were randomly selected from the 1,103,679 subjects who had no COPD diagnosis in the database. To minimize baseline imbalances and reduce potential confounding, propensity score matching (PSM) was employed to ensure comparability between the COPD and non-COPD groups. Propensity scores were derived using a multivariable logistic regression model, estimating the probability of COPD diagnosis based on a comprehensive set of covariates. These included age, sex, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, end-stage renal disease, hepatitis B, hepatitis C, pneumoconiosis, lung cancer, rheumatoid arthritis, and head and neck cancer. Matching was conducted at a 1:1 ratio using greedy nearest-neighbor matching without replacement, with a caliper of 0.05 on the logit of the propensity score, thereby enhancing the balance of baseline characteristics and mitigating selection bias [12]. Controls outside the region of common support were discarded. Cox proportional hazards models were fitted on the matched cohort with robust sandwich standard errors clustered by matched pair. For each control subject, the index date was defined as the same date as that of the corresponding matched COPD patient, to ensure identical follow-up initiation and to avoid immortal time bias. Standardized mean differences (SMDs) were reported for all covariates pre- and post-matching.
Outcomes and comorbidities
To evaluate the incidence of TB, participants were followed from the index date until the earliest occurrence of one of the following events: a diagnosis of TB (ICD-9-CM codes 010-018), death, withdrawal from the National Health Insurance program, or the end of the observation period on December 31, 2021. Comorbid conditions were identified based on diagnostic codes recorded in outpatient and inpatient claims within 1 year prior to the index date. These comorbidities included diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401-405), hyperlipidemia (ICD-9-CM code 272), coronary artery disease (ICD-9-CM codes 410-414), end-stage renal disease (ICD-9-CM codes 585, 585.6), hepatitis B (ICD-9-CM codes V02.61, 070.20, 070.22, 070.30, 070.32), hepatitis C (ICD-9-CM codes V02.62, 070.41, 070.44, 070.51, 070.54), pneumoconiosis (ICD-9-CM codes 500-505), lung cancer (ICD-9-CM code 162), rheumatoid arthritis (ICD-9-CM code 714), and head and neck cancer (ICD-9-CM codes 146.0-146.9, 149.0-149.1). Each comorbidity was coded as a binary variable (1 = presence, 0 = absence).
Statistical analysis
Descriptive statistics for demographic and comorbidity variables in both COPD and non-COPD groups were presented as frequencies with percentages for categorical variables and as means with standard deviations (SDs) for continuous variables. Group comparisons were conducted using the Chi-square test for categorical variables and Student’s t-test for continuous variables. Demographic characteristics included sex and age, stratified into four groups: 40 - 49, 50 - 59, 60 - 69, and ≥ 70 years. The cumulative incidence of TB was estimated using the Kaplan-Meier method, and differences between groups were assessed with the log-rank test. TB incidence rates were calculated as the number of TB events per 10,000 person-years of follow-up. To evaluate the association between comorbidities and the risk of TB among COPD patients, Cox proportional hazards models were applied. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were estimated. Variables found to be statistically significant in univariable analyses were included in the multivariable Cox regression model. All statistical tests were two-tailed, and a P-value < 0.05 was considered indicative of statistical significance. Analyses were conducted using the R statistical software environment.
| Results | ▴Top |
Patient characteristics
A total of 117,989 patients with COPD met the eligibility criteria and were included in the study cohort (Fig. 1). Propensity scores were computed based on covariates associated with TB risk for all participants. Following 1:1 propensity score matching, an equal number of 117,989 individuals without COPD were selected as the control cohort. Among the COPD group, 221 patients (0.19%) developed TB, compared to 176 patients (0.15%) in the non-COPD group (Table 1). Baseline characteristics of both cohorts, including age, sex, and relevant comorbidities, are presented in Table 1. SMDs were computed for all covariates before and after matching. Post-matching balance met conventional criteria (all |SMD| < 0.10), indicating adequate covariate balance between groups, supporting the comparability of the matched cohorts.
 Click to view | Table 1. Demographic Characteristics and Comorbidities of Patients With and Without COPD |
Incidence of TB
Table 2 compares COPD vs. non-COPD within each comorbidity. The overall incidence rate of TB was significantly higher in the COPD cohort compared to the non-COPD cohort (3.20 vs. 1.45 per 10,000 person-years). After adjusting for potential confounders including age and gender, the risk of developing TB remained significantly elevated in the COPD group, with an adjusted hazard ratio (aHR) of 1.74 (95% CI: 1.423 - 2.135; P < 0.001), indicating a 74% increased risk relative to individuals without COPD. Further stratified analysis revealed that the incidence rates of TB in the COPD population increased progressively with age: 1.45, 2.37, 2.80, and 4.51 per 10,000 person-years for age groups 40 - 49, 50 - 59, 60 - 69, and ≥ 70 years, respectively - rates that were consistently higher than those observed in the non-COPD cohort across all age strata. Cox proportional hazards analysis identified age, sex, and comorbid conditions as significant factors associated with increased TB risk in the COPD group compared to the non-COPD group. For instance, among male patients, the aHR for TB was 1.80 (95% CI: 1.41 - 2.30; P < 0.001) in the COPD group, further highlighting the amplified risk relative to their non-COPD counterparts. To minimize protopathic bias, a sensitivity analysis was performed by excluding TB cases diagnosed within 6 months after the index date. The results were consistent with the main analysis.
 Click to view | Table 2. Incidence of Tuberculosis in Patients With and Without COPD |
Comorbidities on TB occurred
The relationship between individual comorbidities and the risk of TB among COPD patients was further examined, as presented in Table 3. The covariates included age, sex, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, and lung cancer, thereby providing aHRs for TB risk. The reference group consisted of COPD patients without any comorbid conditions, allowing for direct comparisons with those who had specific comorbidities. Significantly elevated risks of TB were observed among COPD patients with pneumoconiosis (aHR: 6.94; 95% CI: 2.72 - 17.71; P < 0.001), lung cancer (aHR: 1.81; 95% CI: 1.07 - 3.05; P = 0.027), and rheumatoid arthritis (aHR: 2.05; 95% CI: 1.01 - 4.12; P = 0.046), indicating that these conditions may substantially contribute to TB susceptibility within this population.
 Click to view | Table 3. Impact of Comorbidities on Tuberculosis Among COPD Patients (n = 117,989) |
Cumulative incidence rates of TB
The cumulative incidence curves depicting TB occurrence over the follow-up period demonstrated a statistically significant difference between the COPD and non-COPD groups (log-rank test, P < 0.05) as illustrated in Figure 2. Further stratified analyses revealed that COPD patients with comorbid pneumoconiosis exhibited a significantly greater risk of developing TB compared to non-COPD patients with pneumoconiosis (log-rank test, P < 0.05; Fig. 3). Comparable elevated risks were also observed among COPD patients with lung cancer (Fig. 4) and rheumatoid arthritis (Fig. 5), indicating these comorbidities amplify TB susceptibility in the COPD population.
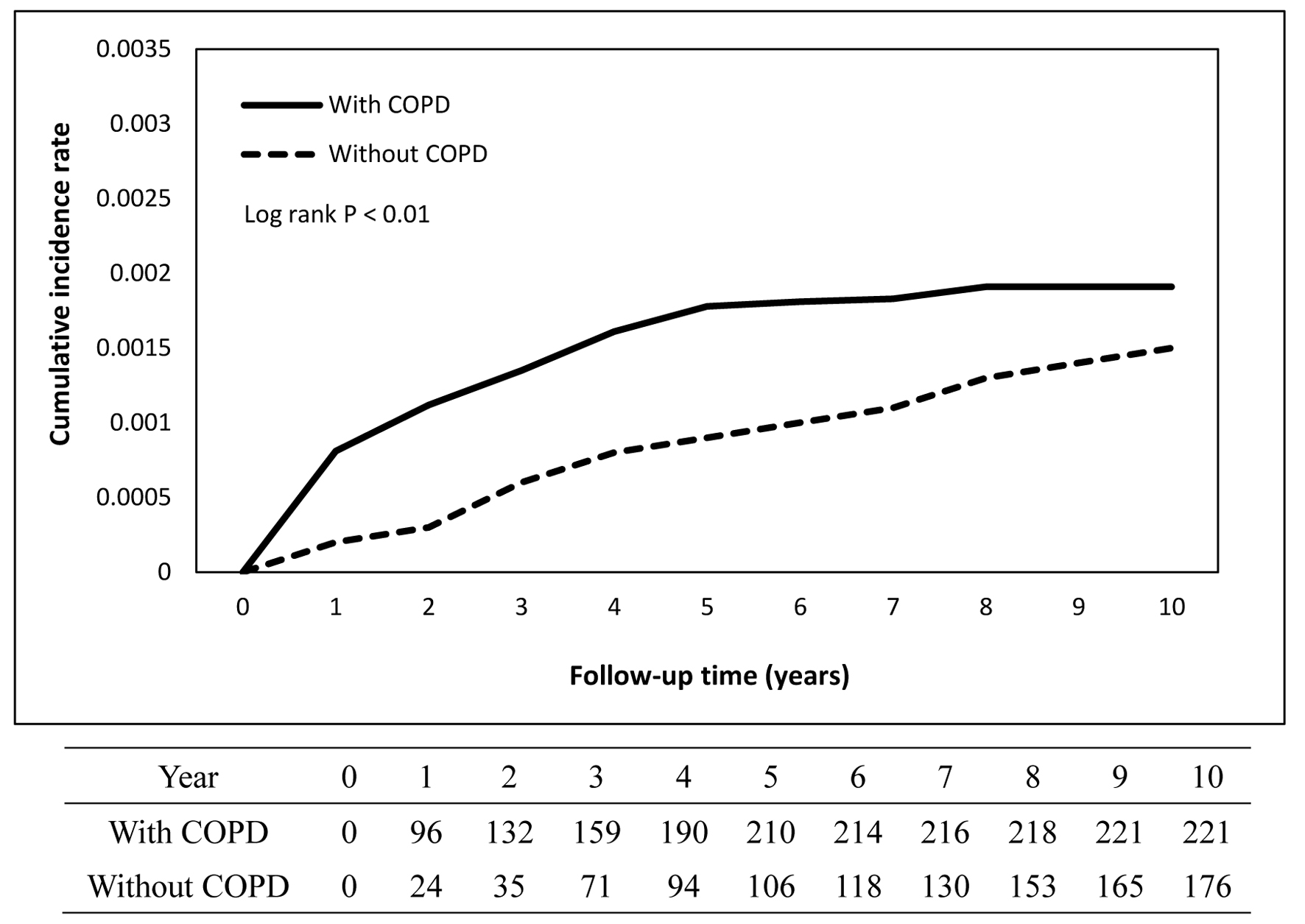 Click for large image | Figure 2. Cumulative incidence rate of tuberculosis in patients with and without COPD. COPD: chronic obstructive pulmonary disease. |
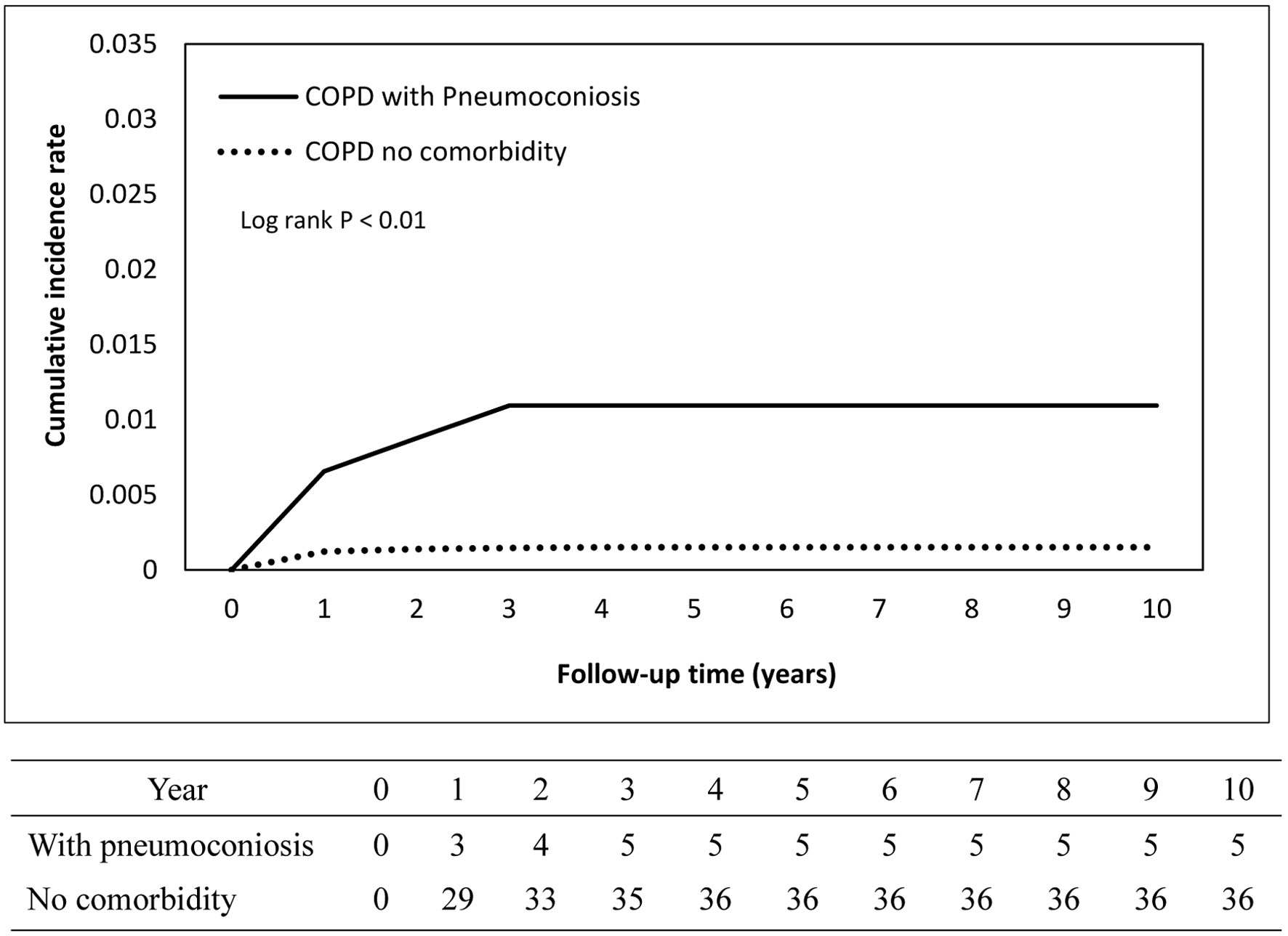 Click for large image | Figure 3. Cumulative incidence rate of tuberculosis in patients with pneumoconiosis. |
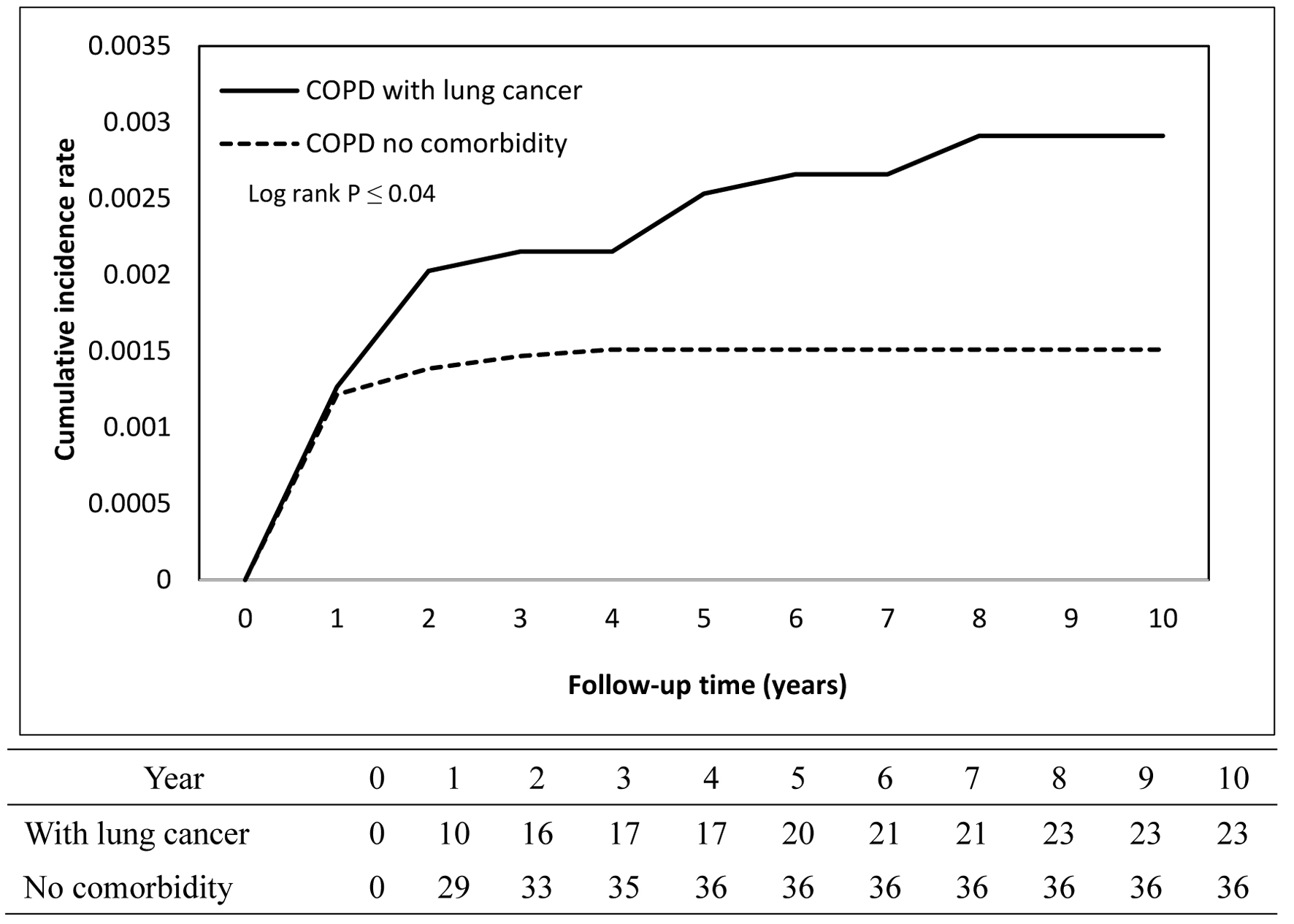 Click for large image | Figure 4. Cumulative incidence rate of tuberculosis in patients with lung cancer. |
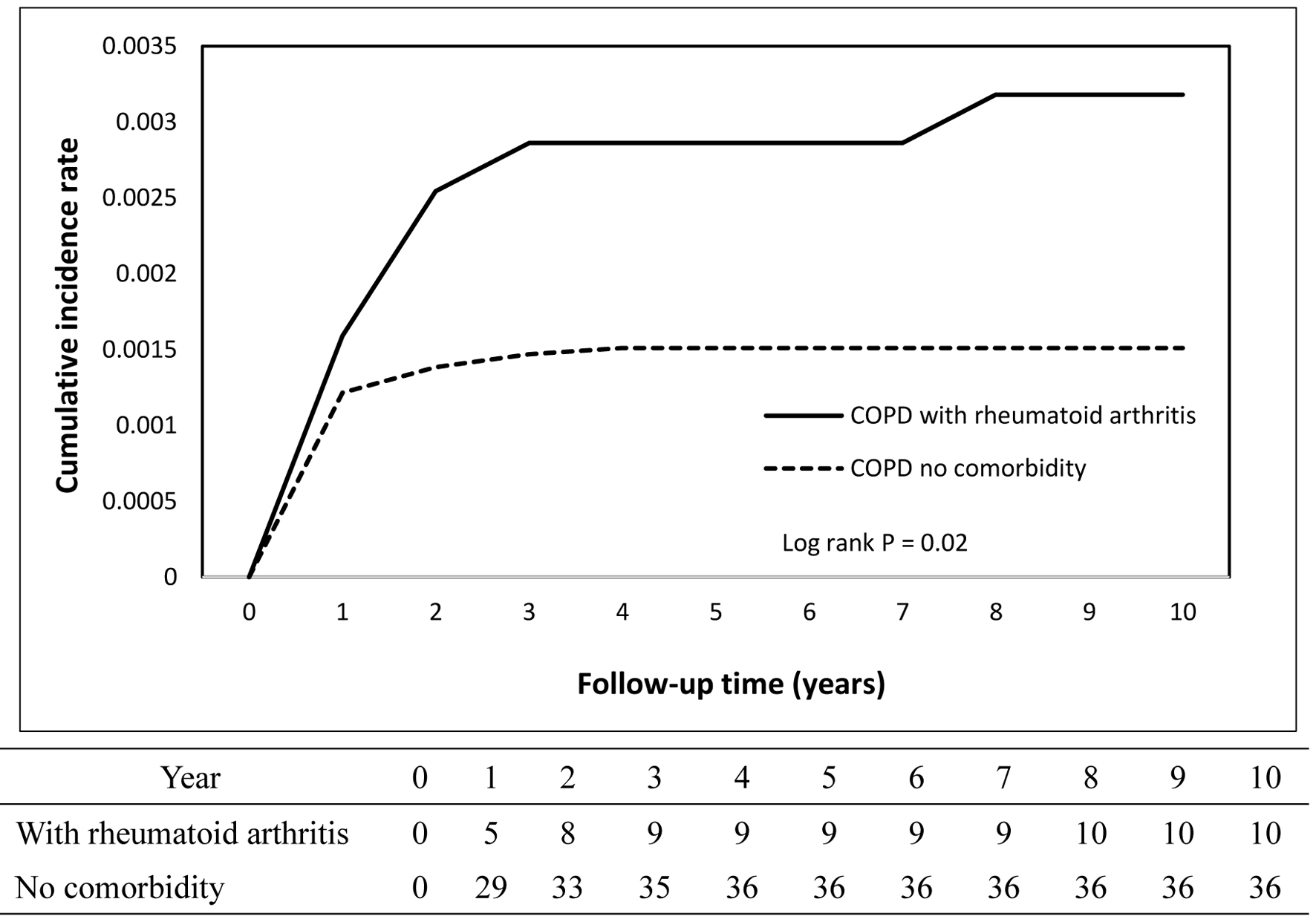 Click for large image | Figure 5. Cumulative incidence rate of tuberculosis in patients with rheumatoid arthritis. |
| Discussion | ▴Top |
From our study, we found that patients with COPD increased the risk of TB. The risk of increased TB was increased with age. The older, the more risk of TB in COPD. The risk also increased with patient comorbidities, especially in pneumoconiosis, rheumatoid arthritis, and lung cancer with aHR: 6.94, 2.05, and 1.81, respectively. In Taiwan, it seems that pneumoconiosis had much higher impact on TB in COPD than other comorbidities.
Three large-scale cohort studies [13-15] have documented a significant association between COPD and an elevated risk of developing TB, even after adjusting for a wide range of potential confounding variables such as age, smoking status, and comorbid conditions. Across these studies, the aHR for TB among patients with COPD ranged from 1.44 to 3.14, all reaching statistical significance, indicating a robust signal that COPD confers a higher likelihood of TB occurrence. Our study found that COPD was associated with an increased risk of developing TB, with an aHR of 1.74. Our finding aligns closely with results from previous research, further reinforcing the evidence that COPD is an independent risk factor for TB across diverse populations and study designs.
Despite the presence of substantial statistical heterogeneity among these investigations - likely attributable to differences in study populations, methodologies, and adjustment strategies - the overall direction of the effect remained consistent, reinforcing the notion that COPD is a risk factor for TB. In contrast, findings from a perspective study conducted by Yii et al [16] demonstrated that chronic bronchitis, a clinical phenotype of COPD, did not exhibit a statistically significant association with TB risk (HR 0.95; 95% CI: 0.68 - 1.31). Another case-control study [17] reported that both chronic bronchitis and emphysema were significantly associated with an elevated risk of developing TB, highlighting that different clinical phenotypes within the spectrum of COPD may contribute to this increased susceptibility.
This suggests that the relationship between COPD and TB may vary depending on the specific respiratory condition and diagnostic criteria applied, the presence of heterogeneity across studies, including variations in study design, population characteristics, diagnostic criteria, and adjustment for confounding factors.
We suggest that there is a significant increase in the risk of TB among individuals with COPD. While the causal relationship between COPD and the subsequent development of active TB remains uncertain, several plausible hypotheses have been proposed to explain this association. One theory was impaired cellular immunity and dysfunctional macrophage activity, both of which are commonly observed in people with COPD, particularly those with a history of long-term smoking [18]. These immune alterations may compromise the body’s ability to eradicate Mycobacterium tuberculosis, thereby increasing vulnerability to infection. Furthermore, smoking itself is a well-established risk factor for both COPD and TB, serving as a shared etiological pathway that may partially account for the observed link between the two conditions [19].
Silicosis is a distinct form of pneumoconiosis, a group of occupational lung diseases caused by the inhalation of dusts. Among the various types of pneumoconiosis, silicosis stands out as one of the strongest and most well-documented risk factors for TB. The coexistence of these two conditions is referred to as silico TB, a serious clinical entity associated with significant morbidity. Epidemiological data indicate that the incidence of TB in individuals with silicosis is strikingly elevated - approximately 21.8 times higher than that observed in the general population [20]. This markedly increased risk is thought to be driven by silica-induced lung damage and impaired macrophage function.
Recently, a comprehensive meta-analysis that included four cohort studies was conducted to clarify the relationship between silica exposure and the risk of TB. Across these studies, a total of 5,884 participants were analyzed, the vast majority of whom were male (90.63%), with a mean age of 47.7 years. The meta-analysis demonstrated a pooled risk ratio of 1.35, indicating that individuals with silicosis or those exposed to silica dust have a significantly higher likelihood of developing TB compared to those without such exposure [21].
In our study, when looking specifically at patients with COPD who also had pneumoconiosis, the risk of TB was dramatically amplified. Data revealed an aHR of 6.94, meaning that pneumoconiosis increased the risk of developing TB nearly sevenfold in individuals with COPD. These findings underscore the synergistic and compounding effects of silica-related lung disease and COPD in predisposing patients to TB.
The pathway through which silicosis predisposes individuals to TB involves a complex interplay of pathological and immunological mechanisms. First, silica-induced lung damage and immune dysfunction. When silica particles are inhaled, they deposit deep within the lungs, triggering persistent inflammation and progressive fibrosis. This chronic injury directly damages alveolar macrophages. Alveolar macrophages were key immune cells responsible for engulfing and controlling Mycobacterium tuberculosis. Damaged macrophages lose much of their bactericidal capacity, compromising the lungs’ frontline defense against infection. Furthermore, studies have demonstrated that silica exposure creates an intracellular environment that supports the proliferation of Mycobacterium tuberculosis within macrophages. Silica appears to interfere with the cellular mechanisms that normally restrict bacterial replication, effectively enabling the bacteria to multiply unchecked and increasing the risk of active infection. Beyond its direct effects on macrophages, silica also impairs broader cell-mediated immunity. This immune suppression reduces the host’s ability to mount an effective defense against Mycobacterium tuberculosis [22, 23]. In summary, pneumoconiosis initiates chronic lung inflammation and fibrosis, damages macrophages, and suppresses cell-mediated immunity. These combined effects create an environment in which Mycobacterium tuberculosis can thrive, ultimately weakening pulmonary defenses and markedly increasing the risk and prevalence of TB. In addition to the mechanisms already discussed, corticosteroid use and increased hospital exposure play important roles in explaining the elevated risk of TB among COPD patients. Systemic or inhaled corticosteroids are prescribed for COPD management and are known to suppress immune function, which may facilitate either reactivation of latent TB or susceptibility to new infections. Furthermore, frequent hospital admissions among COPD patients may increase opportunities for nosocomial TB exposure, particularly in regions with high TB prevalence.
Screening for latent TB may be warranted in patients with COPD. This could include a combination of diagnostic approaches such as a tuberculin skin test or interferon-gamma release assay, chest radiography, or computed tomography scans. Clinicians should also maintain heightened vigilance for new or worsening clinical symptoms, including persistent or worsening cough, hemoptysis, night sweats, unexplained weight loss, or failure to respond to standard COPD therapy, as these may indicate active TB or reactivation of latent infection.
Our study has several limitations that should be acknowledged when interpreting the findings. First, we were unable to obtain data on certain key risk factors, most notably smoking status. The absence of this information represents an inherent limitation of large database studies, as lifestyle factors are often underreported or unavailable in such datasets. Although the NHIRD lacks information on lifestyle factors such as smoking status and body mass index (BMI), our analyses indirectly accounted for potential smoking-related effects through adjustment for comorbidities such as hypertension, hyperlipidemia, coronary artery disease, and lung cancer. Second, the diagnosis of COPD in our study relied on physician-documented medical records rather than objective measurements, such as spirometry or other pulmonary function tests, which were not captured in the database. This reliance on clinical diagnosis introduces the possibility of misclassification or diagnostic variability. Nonetheless, prior validation studies using similar administrative data sources have demonstrated high reliability and accuracy of COPD diagnoses [24, 25], supporting the credibility of our case definition. Third, because cause-specific mortality data were unavailable in the NHIRD, a Fine-Gray competing risks analysis could not be performed. Therefore, potential overestimation of TB risk due to competing mortality in elderly patients cannot be fully excluded. In our analysis, we did not account for the use, dosage, or duration of inhaled or systemic corticosteroids, nor for healthcare utilization intensity, which could potentially influence the likelihood of TB detection. These unmeasured factors may introduce residual confounding and could potentially bias the detection of TB, thereby limiting the strength of causal inference. To address potential confounding, we conducted a reanalysis excluding patients with autoimmune diseases who received immunosuppressive medications and human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS). The findings remained consistent, suggesting that the small number of patients with HIV/AIDS or autoimmune diseases who received immunosuppressive medications had minimal impact on the overall results. Participants were matched and adjusted for major comorbidities (e.g., diabetes, COPD, malignancy) as well as for the number of outpatient visits and hospitalizations prior to the index date, which served as a proxy for healthcare utilization intensity. Although residual confounding cannot be entirely ruled out, these measures substantially reduce its potential influence, and the overall direction of the association is unlikely to be affected. Despite these limitations, the primary objective of our study was to generate comprehensive, population-level data that could provide a broader understanding of the relationship between COPD and the risk of TB. By leveraging a large, nationally representative dataset, our findings offer valuable insights that are generalizable across all stages and severities of COPD, ultimately contributing to the evidence base needed to guide public health strategies and clinical decision-making.
Conclusions
After adjustment for available covariates, COPD was associated with an increased risk of TB (aHR ≈ 1.74); however, residual confounding due to factors such as corticosteroid use, immunosuppressive therapy, and smoking cannot be excluded. This finding highlights the need for further research to clarify the underlying mechanistic pathways linking COPD and TB, particularly at the cellular and molecular levels, where immune dysregulation and chronic inflammation may play critical roles. Clinically, these results underscore the importance of maintaining a high index of suspicion for TB in patients with COPD, ensuring timely diagnosis and appropriate treatment to mitigate the impact of TB on this vulnerable population. Strengthening TB detection and management efforts may ultimately reduce the future disease burden and contribute to more comprehensive care for COPD.
Acknowledgments
The authors express their sincere gratitude to Ms. Ting-Hsing Yeh (Department of Industrial Engineering and Management, National Yunlin University of Science and Technology) for her efforts in organizing data and assisting statistical analysis.
Financial Disclosure
This work was supported by a grant from the Chi Mei Medical Center, Chali, Taiwan (CCFHR11403).
Conflict of Interest
Authors have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: KML, LTK, and HYL; data curation: HYL; formal analysis: HYL; funding acquisition: KML; investigation: LTK and HYL; methodology: KML, LTK, and HYL; project administration: LTK and HYL; resources: HYL; software: HYL; writing - original draft: KML and HYL; writing - review and editing: KML and HYL.
Data Availability
Due to its ethical concerns and data protection, supporting data are not made openly available. Further information about the data and permission for access are available at the NHIRD website (https://nhird.nhri.org.tw/en).
| References | ▴Top |
- Hamada Y, Fong CJ, Copas A, Hurst JR, Rangaka MX. Risk for development of active tuberculosis in patients with chronic airway disease-a systematic review of evidence. Trans R Soc Trop Med Hyg. 2022;116(5):390-398.
doi pubmed - Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899-909.
doi pubmed - Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335-342.
doi pubmed - Kanervisto M, Vasankari T, Laitinen T, Heliovaara M, Jousilahti P, Saarelainen S. Low socioeconomic status is associated with chronic obstructive airway diseases. Respir Med. 2011;105(8):1140-1146.
doi pubmed - Gupta D, Das K, Balamughesh T, Aggarwal AN, Jindal SK. Role of socio-economic factors in tuberculosis prevalence. Indian J Tuberc. 2004;51:27-31.
- Choi JY. Pathophysiology, clinical manifestation, and treatment of tuberculosis-associated chronic obstructive pulmonary disease: a narrative review. Ewha Med J. 2025;48(2):e24.
doi pubmed - Aggarwal D, Gupta A, Janmeja AK, Bhardwaj M. Evaluation of tuberculosis-associated chronic obstructive pulmonary disease at a tertiary care hospital: A case-control study. Lung India. 2017;34(5):415-419.
doi pubmed - Fan H, Wu F, Liu J, Zeng W, Zheng S, Tian H, Li H, et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Transl Med. 2021;9(5):390.
doi pubmed - Jiang Z, Dai Y, Chang J, Xiang P, Liang Z, Yin Y, Shen Y, et al. The clinical characteristics, treatment and prognosis of tuberculosis-associated chronic obstructive pulmonary disease: a protocol for a multicenter prospective cohort study in China. Int J Chron Obstruct Pulmon Dis. 2024;19:2097-2107.
doi pubmed - Kumar V, Nazli Khatib M, Verma A, Lakhanpal S, Ballal S, Kumar S, Bhat M, et al. Tuberculosis in South Asia: A regional analysis of burden, progress, and future projections using the global burden of disease (1990-2021). J Clin Tuberc Other Mycobact Dis. 2024;37:100480.
doi pubmed - Kim J, Keshavjee S, Atun R. Health systems performance in managing tuberculosis: analysis of tuberculosis care cascades among high-burden and non-high-burden countries. J Glob Health. 2019;9(1):010423.
doi pubmed - Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med. 2005;24(10):1563-1578.
doi pubmed - Inghammar M, Ekbom A, Engstrom G, Ljungberg B, Romanus V, Lofdahl CG, Egesten A. COPD and the risk of tuberculosis—a population-based cohort study. PLoS One. 2010;5(4):e10138.
doi pubmed - Lee CH, Lee MC, Shu CC, Lim CS, Wang JY, Lee LN, Chao KM. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis. 2013;13:194.
doi pubmed - Park S, Lee S, Kim Y, Lee Y, Kang MW, Cho S, Han K, et al. Association of CKD with Incident Tuberculosis. Clin J Am Soc Nephrol. 2019;14(7):1002-1010.
doi pubmed - Yii AC, Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP. Asthma, sinonasal disease, and the risk of active tuberculosis. J Allergy Clin Immunol Pract. 2019;7(2):641-648.e641.
doi pubmed - Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19-26.
doi pubmed - O'Toole RF, Shukla SD, Walters EH. TB meets COPD: An emerging global co-morbidity in human lung disease. Tuberculosis (Edinb). 2015;95(6):659-663.
doi pubmed - Sarkar M, Srinivasa, Madabhavi I, Kumar K. Tuberculosis associated chronic obstructive pulmonary disease. Clin Respir J. 2017;11(3):285-295.
doi pubmed - Farazi A, Jabbariasl M. Silico-tuberculosis and associated risk factors in central province of Iran. Pan Afr Med J. 2015;20:333.
doi pubmed - Jamshidi P, Danaei B, Arbabi M, Mohammadzadeh B, Khelghati F, Akbari Aghababa A, Nayebzade A, et al. Silicosis and tuberculosis: A systematic review and meta-analysis. Pulmonology. 2025;31(1):2416791.
doi pubmed - Allison AC, Hart PD. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968;49(5):465-476.
pubmed - Shafiei M, Ghasemian A, Eslami M, Nojoomi F, Rajabi-Vardanjani H. Risk factors and control strategies for silicotuberculosis as an occupational disease. New Microbes New Infect. 2019;27:75-77.
doi pubmed - Liao KM, Lee CS, Wu YC, Shu CC, Ho CH. Prior treated tuberculosis and mortality risk in lung cancer. Front Med (Lausanne). 2023;10:1121257.
doi pubmed - Liu HY, Liao KM, Liang FW, Hung YC, Wang JJ, Shen TC, Ho CH. The risk of pulmonary tuberculosis after traumatic brain injury. Sci Rep. 2021;11(1):7840.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.