| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 518-528
Clinical Significance of the Cardio-Ankle Vascular Index as a Cardiovascular Disease Risk Factor in Japanese Elderly Patients With Obesity
Takashi Hitsumoto
Hitsumoto Medical Clinic, Shimonoseki City, Yamaguchi 750-0025, Japan
Manuscript submitted August 8, 2025, accepted September 12, 2025, published online October 10, 2025
Short title: CAVI as a CVD Risk in Elderly With Obesity
doi: https://doi.org/10.14740/jocmr6351
| Abstract | ▴Top |
Background: The cardio-ankle vascular index (CAVI) is a physiological marker that indicates systemic arterial stiffness, and several reports have demonstrated its usefulness as a predictor of cardiovascular disease (CVD). However, there have been no studies examining the clinical significance of CAVI limited to elderly patients with obesity. This prospective study aimed to determine the clinical significance of CAVI as a CVD risk factor in Japanese elderly patients with obesity.
Methods: This study included a total of 402 Japanese elderly patients with obesity (mean age ± standard deviation: 72 ± 5 years; mean body mass index ± standard deviation: 27.6 ± 2.2 kg/m2) and no history of CVD events. The patients were divided into two groups: the high CAVI group (group H: n = 193) and the low CAVI group (group L: n = 209) (cut-off value: 9.0). The patients’ background factors were compared between groups, and the usefulness of the CAVI as a predictor of primary CVD events was assessed.
Results: Group H showed a significantly higher visceral fat area, as measured by abdominal computed tomography, compared to group L. A significant relation with biomarkers, such as homeostasis model assessment of insulin resistance (HOMA-IR); preheparin serum lipoprotein lipase mass (pre-LPL mass) concentration, one of the coronary risk factors; and urinary 8-iso-prostaglandinF2α (U-8-iso-PGF2α) concentration, an indicator of oxidative stress in vivo, was observed. The multiple Cox proportional hazards regression analysis for the incidence of primary CVD events (median follow-up period: 100 months) indicated that group H had a significantly higher risk of developing primary CVD events than group L (hazard ratio: 2.40; 95% confidence interval: 1.37 - 4.24; P = 0.002). However, high HOMA-IR, low pre-LPL mass, and high U-8-iso-PGF2α were also selected as significant predictors of primary CVD events.
Conclusions: The results of this study indicated that the CAVI increases in the presence of visceral fat accumulation and is significantly associated with key CVD risk factors, such as insulin resistance, low pre-LPL mass, and elevated oxidative stress in Japanese elderly patients with obesity. Furthermore, high CAVI is considered a useful predictor of primary CVD events in such patients.
Keywords: Cardio-ankle vascular index; Elderly patients with obesity; Visceral fat accumulation; Insulin resistance; Preheparin serum lipoprotein lipase mass; Oxidative stress; Primary cardiovascular disease events
| Introduction | ▴Top |
Recent epidemiological studies have reported that there are many people with obesity, and that a certain proportion of elderly individuals also have obesity [1]. In daily clinical practice, clinicians encounter many elderly patients with obesity. Several studies have also investigated the relationship between elderly patients with obesity and classical cardiovascular disease (CVD) risk factors, such as hypertension, dyslipidemia, and diabetes mellitus [2, 3]. In fact, even in daily practice, elderly patients complicated by obesity with a classical CVD risk factor are common. However, despite the standard medical treatments for these classical CVD risk factors, such as blood pressure-lowering therapy, blood glucose-lowering therapy, and serum lipid-lowering therapy, a certain number of patients would still develop CVD events. Thus, if a novel CVD risk factor in addition to the classical CVD risk factor can be identified and efficiently intervened in elderly patients with obesity, it can facilitate the prevention of further CVD development, making it clinically meaningful.
The cardio-ankle vascular index (CAVI) was developed as a physiological marker of systemic arterial stiffness that is independent of blood pressure at the time of measurement [4]. In recent years, basic and clinical studies have confirmed the significance of the CAVI as a CVD risk [5-9]. Furthermore, several prospective studies have demonstrated that CAVI is an important indicator for developing CVD [10-14]. Satoh-Asahara et al reported that CAVI can predict CVD events in Japanese patients with obesity with a mean age of 51.5 years [12]. Therefore, the existing study is considered as mainly targeting the middle-aged population. However, to our knowledge, there have been no studies examining the analysis of CAVI limited to elderly patients with obesity, and furthermore, the usefulness of CAVI as a predictor of primary CVD events in such patients has not been validated. Therefore, this prospective study tried to determine the clinical significance of CAVI as a CVD risk factor in Japanese elderly patients with obesity using various analysis.
| Materials and Methods | ▴Top |
Patients
Inclusion criteria were defined as 452 consecutive Japanese elderly patients with obesity (age ≥ 65 years and body mass index (BMI) ≥ 25.0 kg/m2) without a history of CVD events, who visited Hitsumoto Medical Clinic in Yamaguchi Prefecture between April 2009 and March 2011. Exclusion criteria included cases in which informed consent could not be obtained (14 cases), cases with insufficient data necessary for this study (14 cases), cases with ankle- brachial index < 0.9 (10 cases), and cases in which atrial fibrillation was present during CAVI measurement (12 cases). Finally, 402 patients (male/female ratio: 147/255; mean age ± standard deviation: 72 ± 5 years; mean BMI ± standard deviation: 27.6 ± 2.2 kg/m2) were enrolled in this study. The BMI of 25.0 kg/m2 or higher, which was used to define Japanese obesity in this study, was determined with reference to existing reports [15].
Ethical considerations
This study was performed in accordance with the Declaration of Helsinki and the ethical principles of medical research involving human beings. Informed consent was obtained from all patients, and the study protocol was approved by the Institutional Review Board of Hitsumoto Medical Clinic (date of approval: March 18, 2009; approval number: HMC-2009- 3).
CAVI measurement
CAVI was measured using a VaSera CAVI instrument (Fukuda Denshi, Tokyo, Japan) based on the previously described methods [4, 16]. In brief, the pulse waves of the upper arm and ankle were determined using an inflatable cuff in which the pressure was maintained at 30 - 50 mm Hg. The systemic blood pressure and pulse pressure were measured simultaneously after the patient had rested in the supine position for 10 min. The formula for measuring the CAVI is as follows: CAVI = a((2ρ/ΔP) × ln (Ps/Pd) × (pulse wave velocity)2) + b, where a and b are constants, ρ is blood density, ΔP is Ps - Pd, Ps is systolic blood pressure, and Pd is diastolic blood pressure. Furthermore, the average coefficient of variation is < 5%, which indicates that the CAVI has good reproducibility [4].
Evaluation of the clinical parameters
Smokers were defined as patients who had smoking habits at baseline. In this study, the classical risk factors for CVD - hypertension, dyslipidemia, and diabetes mellitus - were defined according to a previous report [11]. The abdominal computed tomography examination measured the visceral fat area (VFA) and subcutaneous fat area at the umbilical level. The blood and urine data were measured in the fasting state. The contents of the data are as follows: serum lipid concentration, blood glucose concentration, blood insulin concentration, preheparin serum lipoprotein lipase mass (pre-LPL mass) concentration, and urinary 8-iso- prostaglandin F2α (U-8-iso-PGF2α) concentration, an in vivo indicator of oxidative stress. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as a marker of insulin resistance by an existing report (HOMA-R = blood glucose concentration × blood insulin concentration/405) [17]. Furthermore, oral medications, such as renin-angiotensin system (RAS) inhibitors, calcium blocker, statin, fibrate, and sulfonylurea were also investigated. None of insulin users were observed in this study
Determination of the primary CVD events and grouping
This study evaluated whether the enrolled patients developed primary CVD events during the observation period, which lasted until March 2025. Moreover, primary CVD events were defined as a composite of cardiovascular death, the development of coronary artery disease requiring invasive and/or medical treatment, and nonfatal ischemic stroke. The patients were divided into two groups based on the optimal cut-off value of the CAVI (cut-off value: 9.0), determined by receiver operating characteristic curve analysis for primary CVD events (Fig. 1): high CAVI group (group H: n = 193) and a low CAVI group (group L: n = 209).
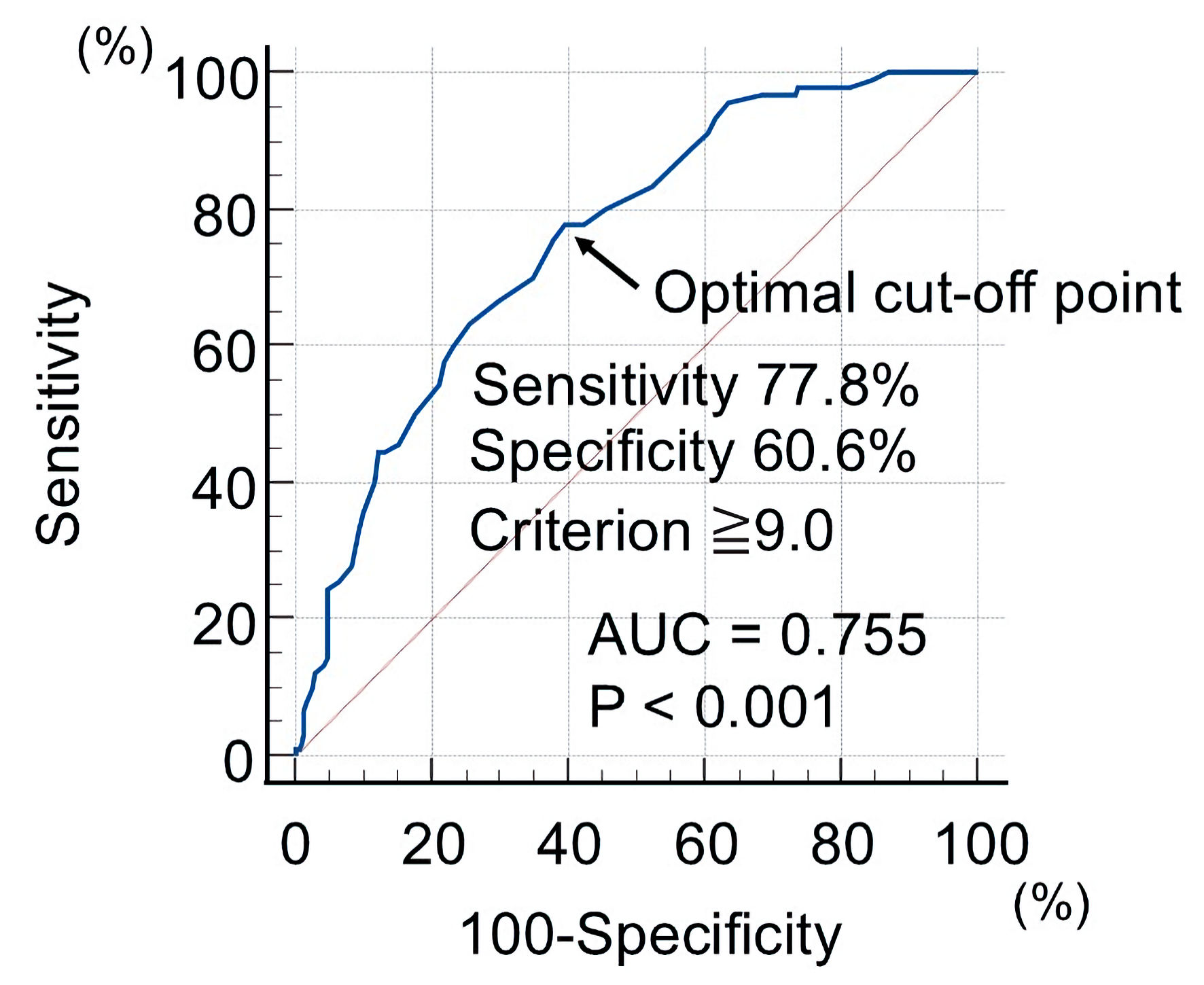 Click for large image | Figure 1. Optimal cut-off value of CAVI to detect primary CVD events using receiver operating characteristic curve analysis. The patients were divided into two groups based on the optimal cut-off value of the CAVI (cut-off value: 9.0): high CAVI group (group H) and a low CAVI group (group L). CAVI: cardio-ankle vascular index; CVD: cardiovascular disease; AUC: area under the curve. |
Statistical analysis
Statistical analysis was performed using the commercialized software Stat View-J 5.0 (HULINKS Inc., Tokyo, Japan) and MedCalc (MedCalc Software, Ostend, Belgium). The optimal cut-off value of the CAVI for primary CVD events was decided via the receiver operating characteristic curve analysis using the Youden index [18]. Continuous variables were expressed as means and standard deviations. Group comparisons were conducted using the unpaired t-test or Mann-Whitney U test, depending in the data distribution. Kaplan-Meier analysis was performed to produce event-free survival curves; with differences between these curves assessed using the log-rank test. Multivariate analysis was performed using multiple Cox proportional hazards regression. Moreover, in this study, the selection of the explanatory variables for the occurrence of primary CVD events in the multiple Cox proportional hazards regression analysis was based on the number of primary CVD events (90 cases), clinical indices that showed the significant differences between the two groups for primary CVD events, based on clinical rationale, and multicollinearity. Finally, nine explanatory variables (i.e., CAVI, age, VFA, diabetes mellitus, HOMA-IR, pre-LPL mass, U-8-iso-PGF2α, RAS inhibitor use, and statin use) were determined. Cut-off values of age, VFA, HOMA-IR, pre-LpL mass, and U-8-iso-PGF2α were decided by receiver operating characteristic curve analysis. In this study, it was determined that the results drawn are statistically significant when the P value is less than 0.05.
| Results | ▴Top |
Baseline clinical characteristics
Table 1 presents the baseline clinical characteristics of the study population. The mean CAVI for groups H and L were 10.6 and 8.1, respectively. The BMI was significantly lower in group H than in group L. However, the VFA was significantly higher in group H than in group L. Regarding CVD risk factors, the frequency of diabetes mellitus, blood glucose concentration, HOMA-IR, and U-8-iso-PGF2α were significantly higher in group H than in group L. However, the high-density lipoprotein cholesterol concentration and pre-LPL mass were significantly lower in group H than in group L. With regard to the medications, group H had a significantly lower frequency of using RAS inhibitors and statin than group L.
 Click to view | Table 1. Baseline Clinical Characteristics |
Kaplan-Meier curve analysis of all patients who participated in this study
Figure 2 shows the results of the Kaplan-Meier curve analysis of all patients who participated in this study regarding the occurrence of primary CVD events. The median observation period was 100 months (range: 2 - 192 months). During the observation period, 90 patients experienced primary CVD events (group L, n = 20 (9.6%), vs. group H, n = 70 (36.3%)). Thus, group H had a significantly higher incidence of primary CVD events than group L (P < 0.001, log-rank test).
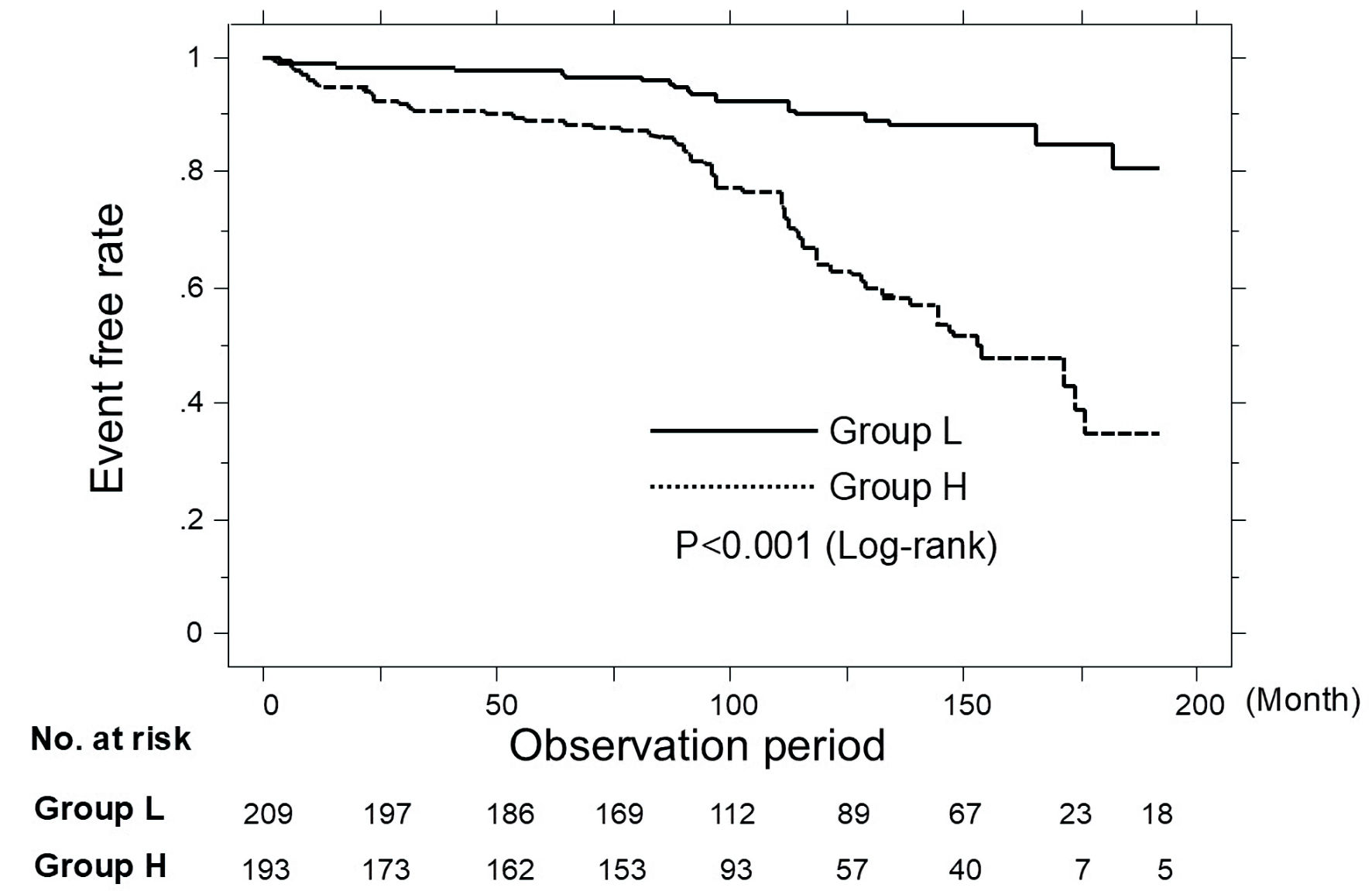 Click for large image | Figure 2. Kaplan-Meier curve analysis for the incidence of primary CVD events in all patients. During the observation period, group H had a significantly higher incidence of primary CVD events than group L (P < 0.001, log-rank test). CVD: cardiovascular disease. |
The association between the presence or absence of primary CVD events and clinical characteristics
Table 2 shows the association between the presence or absence of primary CVD events and clinical characteristics in this study. BMI was similar in patients with and without primary CVD events. Age, VFA, frequency of diabetes mellitus, fasting blood glucose concentration, HOMA-IR, and U-8-iso-F2α were significantly higher in patients who experienced primary CVD events than in patients who did not. However, the patients who experienced primary CVD events had significantly lower pre-LPL mass, RAS inhibitor use, and statin use than those who did not.
 Click to view | Table 2. Clinical Characteristics at Registration of Patients With and Without Cardiovascular Disease Events |
Kaplan-Meier curve analysis in patients not using RAS inhibitor and statin
Figure 3 shows the results of the Kaplan-Meier curve analysis in patients not using RAS inhibitor and statin regarding the occurrence of primary CVD events. During the observation period, 47 patients experienced primary CVD events (group L, n = 9 (17.6%), vs. group H, n = 38 (49.4%)). Thus, group H had a significantly higher incidence of primary CVD events than group L (P =0.013, log-rank test).
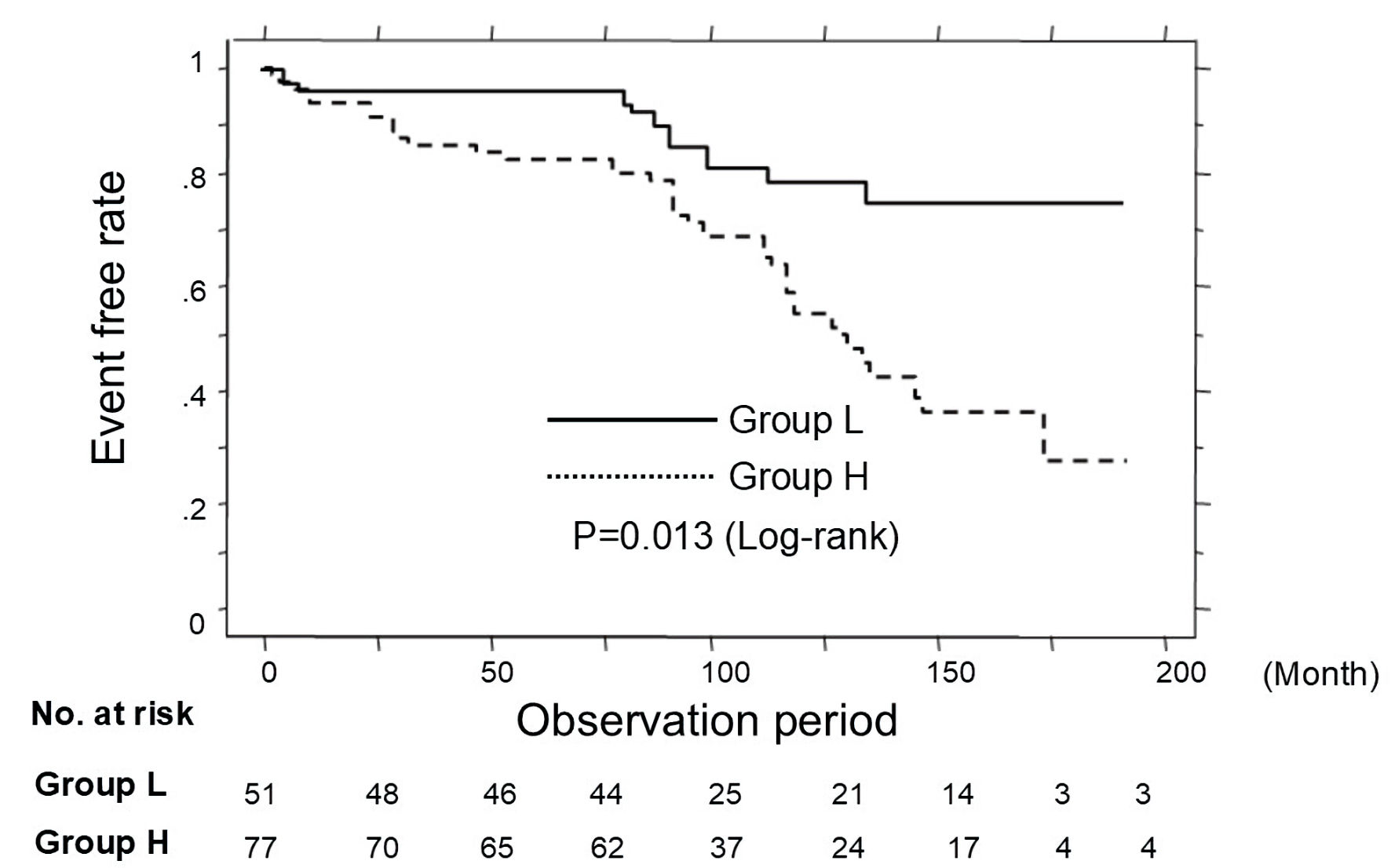 Click for large image | Figure 3. Kaplan-Meier curve analysis in patients not using RAS inhibitor and statin. During the observation period, group H had a significantly higher incidence of primary CVD events than group L (P = 0.013, log-rank test). CVD: cardiovascular disease. |
Multivariate Cox regression analysis
Table 3 shows the results of the multivariable Cox regression analysis for the onset of primary CVD events. Even after being adjusted for covariates, group H had a significantly higher risk of developing primary CVD events than those in group L (hazard ratio: 2.40; 95% confidence interval: 1.37 - 4.24; P = 0.002). With regard to the other factors, six variables (i.e., RAS inhibitor use, low pre-LPL mass, high HOMA-IR, aging, statin use, and high U-8-iso-PGF2α) also showed significant hazard ratios for the onset of primary CVD events. In contrast, the presence of diabetes mellitus and high VFA were not considered as significant variables for the occurrence of primary CVD events.
 Click to view | Table 3. Multivariate Cox Regression Analysis for Cardiovascular Disease Events |
| Discussion | ▴Top |
This study aimed to investigate the clinical significance of CAVI as a CVD risk factor in Japanese elderly patients with obesity. When comparing baseline clinical backgrounds, patients with a high CAVI exhibited significantly higher VFA, as measured by abdominal computed tomography, compared to those with a low CAVI. Moreover, high CAVI was significantly associated with several independent risk factors for primary CVD events identified in the multivariable Cox regression analysis, including insulin resistance, low pre-LPL mass, and elevated oxidative stress. Importantly, high CAVI itself was also considered an independent predictor of primary CVD events. Several clinical studies have shown that CAVI is an important predictor of primary CVD events such as patients with cardiovascular risk factors, chronic kidney disease, metabolic syndrome cases, participants of the Malaysian Cohort, and middle-aged patients with obesity [10-14]. However, the findings obtained in this study represent the first report demonstrating that CAVI is an independent predictor of primary CVD events in elderly patients with obesity. In addition, it has been revealed that a single time-point measurement of CAVI, together with several biomarkers at baseline, was found to predict subsequent primary CVD events. However, patients with a high CAVI had a significantly lower frequency of using medications selected as significant inhibitory factors for primary CVD events in this study, such as statins and RAS inhibitors, compared with those with a low CAVI.
Adiposity, diabetes mellitus, and CAVI
Several studies have shown a negative correlation between CAVI and BMI [19, 20]. In the study limited to elderly patients with obesity, those with a high CAVI had a significantly lower BMI compared to those with a low CAVI, confirming the negative association between CAVI and BMI. However, in the studies investigating the relationship between VFA and CAVI, the patients with a high CAVI had a significantly higher VFA than those with a low CAVI. A significant association between CAVI and visceral fat accumulation has been reported in existing research [21, 22]. In this study, similar results in elderly patients with obesity were also observed. Several studies have found that visceral fat accumulation is an important risk factor for CVD [23-25]. In the present study, the VFA of the patients who developed primary CVD events was significantly higher than that of those who did not. However, in the multivariable Cox regression analysis, the VFA was not selected as a significant predictor of primary CVD events. While the importance of visceral fat accumulation in CVD onset is an undeniable fact, the novel insights gained from this study suggest that the increase in the CAVI underlying visceral fat accumulation plays an important role in the onset of primary CVD events in elderly patients with obesity.
Several studies have confirmed that diabetes mellitus is closely related to obesity and is an important risk factor for the onset of CVD [2, 3, 26, 27]. Moreover, it has been reported that patients with diabetes mellitus tend to have a significantly higher CAVI compared to those with normoglycemia [28]. However, among the elderly patients with obesity in the present study, the proportion of patients with diabetes mellitus among those who developed primary CVD events was significantly higher than that of those who did not develop primary CVD events. In addition, the proportion of patients with diabetes mellitus among those with a high CAVI was also significantly higher than that of those with a low CAVI. Thus, these results are consistent with existing reports. However, the multivariable Cox regression analysis did not consider diabetes mellitus as a significant predictor of primary CVD events. Thus, the results of this study suggest that in elderly patients with obesity, the increase in the CAVI underlying the presence of diabetes mellitus is closely related to the onset of primary CVD events, and it is important to actively intervene with the treatment aimed at reducing the CAVI for the prevention of primary CVD events in elderly patients with obesity and diabetes mellitus.
Biomarker and CAVI
Basic and clinical studies have indicated that insulin resistance plays a key role in the progression of atherosclerosis [29, 30]. In the present study, HOMA-IR, an indicator of insulin resistance, was selected as an independent predictor of primary CVD events in elderly patients with obesity, suggesting the importance of insulin resistance in the development of primary CVD events in this population. Moreover, several studies have reported a significant association between insulin resistance and CAVI [16, 31, 32]. The present study also found that patients with a high CAVI had a significantly higher HOMA-IR compared with those with a low CAVI, which is consistent with the findings of existing reports. These results suggest that improving insulin resistance, primarily through lifestyle modifications such as diet and exercise, can help prevent primary CVD events in elderly patients with obesity by reducing CAVI. However, recent prospective studies have shown that pioglitazone, a drug used for improving insulin resistance, significantly reduced the occurrence of CVD events [33, 34]. In addition, Ohira et al reported that pioglitazone improves CAVI in patients with type 2 diabetes [35]. Unfortunately, no cases of pioglitazone administration in the study population were observed, making it impossible to determine whether pioglitazone could be involved in the prevention of primary CVD events in elderly patients with obesity. However, based on previous findings, it is expected that pioglitazone could contribute to the prevention of primary CVD events in elderly patients with obesity and high CAVI. Further research is needed to confirm whether this can be achieved by preventing primary CVD events through a reduction in the CAVI.
Several studies have suggested that the pre-LPL mass present in the blood prior to heparin intravenous injection shows lower values in the state of visceral fat accumulation [36], and it has been considered as an important risk factor for CVD, particularly coronary artery disease [37-39]. Moreover, in the present study which specifically targets elderly individuals with obesity, the pre-LPL mass was selected as an independent predictor of the onset of primary CVD events, highlighting its importance as a risk factor for CVD in elderly patients with obesity. However, the pre-LPL mass of the patients with a high CAVI was significantly lower than those with a low CAVI. Moteki et al similarly reported a negative correlation between the CAVI and pre-LPL mass, which confirms the findings of this study [40]. Saiki et al reported that the lipoprotein lipase derived from subcutaneous fat is involved in the reduction of CAVI in patients with visceral fat accumulation [41]. This fact suggests that the lipoprotein lipase in subcutaneous fat exhibits an atheroprotective effect. Thus, the low pre-LPL mass in this study reflects the pathogenesis of visceral fat accumulation, highlighting the importance of targeting visceral fat reduction in the treatment of elderly patients with obesity to prevent primary CVD events from the perspective of in vivo lipoprotein lipase activity.
Several studies have reported that oxidative stress plays an important role in the onset of CVD [42, 43]. In the present study, U-8-iso-PGF2α, a representative oxidative stress marker, was selected as an independent factor predicting primary CVD events in elderly patients with obesity. However, the levels of U-8-iso-PGF2α in patients with a high CAVI were significantly higher than those in patients with a low CAVI. Several researchers have shown a significant association between an elevated CAVI and oxidative stress [44, 45], which is consistent with the results of the present study. Thus, the treatments that reduce oxidative stress may facilitate the prevention of primary CVD events through a reduction in the CAVI.
Statin, RAS inhibitor and CAVI
Numerous clinical studies have confirmed that statin and RAS inhibitors significantly reduce the CVD risk [46, 47]. In the present study, these medications were also associated with a significant reduction in the onset of primary CVD events among elderly patients with obesity. However, the frequency of using both medications in the high CAVI group was significantly lower than that in the low CAVI group. Several clinical studies have reported that both medications significantly reduce CAVI. Miyashita et al reported that pitavastatin, a type of statin, significantly decreased oxidative stress markers, such as urinary 8-hydroxy-2'-deoxyguanosine, serum malondialdehyde-low density lipoprotein, and CAVI [48]. They also reported that olmesartan, which is one of the RAS inhibitors, showed a significant relationship between the decrease in CAVI and the decrease in urinary 8-hydroxy-2'-deoxyguanosine due to the medication [49]. Moreover, it has been suggested that both medications exhibit an effect of increasing the pre-LPL mass [50-52]. In addition, in the Kaplan-Meier curve analysis of this study, not only was there a significant occurrence of primary CVD events in the high CAVI group compared to the low CAVI group among all subjects, but also in cases that were not taking statin or RAS inhibitor. This suggests that, actively using both drugs in high CAVI cases not taking statins or RAS inhibitors may help suppress primary CVD events in elderly patients with obesity through the pathway of oxidative stress suppression and increase of pre-LpL mass.
Cut-off value of CAVI
In this study, in determining the cut-off value of CAVI for elderly patients with obesity, a receiver operating characteristic curve analysis for primary CVD events was used, resulting in a value of 9.0. The cut-off value of 9.0 for CAVI has also been indicated in existing reports [14, 53]. However, in this study, the cut-off value of 9.0 for CAVI also shows statistically significant results in the multivariate Cox regression analysis for primary CVD events. Therefore, targeting a CAVI value of 9.0 or lower for intervention treatment in elderly patients with obesity suggests the possibility of suppressing primary CVD events in elderly patients with obesity. It is necessary to perform external validation in the future to assess the validity of the cut-off value obtained from this study.
Limitations
This study has several limitations. First, the study is single-center and limited to elderly Japanese patients with obesity. Therefore, the findings may not be extrapolated to populations with different obesity phenotypes or risk factor profiles. Second, this study showed that a high CAVI is an independent predictor in elderly patients with obesity; however, it did not quantify its additional value against existing risk scores (e.g., Framingham or assessment of 10-year atherosclerotic CVD). In the future, it is desired to strengthen the claim of the clinical utility of this study’s results by reporting C statistics, net reclassification improvement, or integrated discrimination improvement. Third, previous studies in Japanese patients have shown that eicosapentaenoic acid (EPA) improves the arterial stiffness, associated with an increased EPA/arachidonic acid (AA) ratio and decreased inflammation in patients with obesity with dyslipidemia [54]. The clinical relevance of decreased ratios of serum EPA/AA and docosahexaenoic acid (DHA)/AA to impaired arterial stiffness has also been reported [55]. However, in the present study, EPA- and DHA-related data were not found. Therefore, further study is required to clarify the clinical significance of CAVI as a CVD risk factor in elderly patients with obesity, particularly from perspective of omega-3 fatty acids. Finally, this study’s results showed that a high baseline CAVI could be a predictive indicator for primary CVD events in elderly patients with obesity. However, it is necessary to investigate the extent to which primary CVD prevention is possible through interventions such as active lifestyle improvements and medications such as pioglitazone, statins, RAS inhibitors, and omega-3 fatty acids in elderly obese patients with a high CAVI.
Conclusions
This study demonstrated that CAVI increased in the situation of visceral fat accumulation and was significantly associated with important CVD risk factors, such as insulin resistance, low pre-LPL mass, and elevated oxidative stress in Japanese elderly patients with obesity. Furthermore, a high baseline CAVI was found to be a useful predictor of primary CVD events in these patients. Considering the limitations of this study, further research is needed to determine the extent to which primary CVD prevention is possible through interventions such as active lifestyle improvements and medications in elderly patients with obesity and high CAVI.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from all patients who participated in this study.
Author Contributions
Takashi Hitsumoto contributed to research planning, data acquisition and analysis, and manuscript writing and editing.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
Abbreviations
AA: arachidonic acid; BMI: body mass index; CAVI: cardio-ankle vascular index; CVD: cardiovascular disease; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; HOMA-IR: homeostasis model assessment of insulin resistance; pre-LpL mass: preheparin serum lipoprotein lipase mass; RAS: renin-angiotensin system; U-8-iso-F2α: urinary 8-iso-prostaglandinF2α; VFA: visceral fat area
| References | ▴Top |
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021. Lancet. 2025;405(10481):813-838.
doi pubmed - Sowers JR. Diabetes in the elderly and in women: cardiovascular risks. Cardiol Clin. 2004;22(4):541-551.
doi pubmed - Yamada T, Kimura-Koyanagi M, Sakaguchi K, Ogawa W, Tamori Y. Obesity and risk for its comorbidities diabetes, hypertension, and dyslipidemia in Japanese individuals aged 65 years. Sci Rep. 2023;13(1):2346.
doi pubmed - Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101-107.
doi pubmed - Okura T, Watanabe S, Kurata M, Manabe S, Koresawa M, Irita J, Enomoto D, et al. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res. 2007;30(4):335-340.
doi pubmed - Nakamura K, Tomaru T, Yamamura S, Miyashita Y, Shirai K, Noike H. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72(4):598-604.
doi pubmed - Hitsumoto T. Relationships between the cardio-ankle vascular index and pulsatility index of the common carotid artery in patients with cardiovascular risk factors. J Clin Med Res. 2019;11(8):593-599.
doi pubmed - Birudaraju D, Cherukuri L, Kinninger A, Chaganti BT, Haroun P, Pidikiti S, Lakshmanan S, et al. Relationship between cardio-ankle vascular index and obstructive coronary artery disease. Coron Artery Dis. 2020;31(6):550-555.
doi pubmed - Chiba T, Yamanaka M, Takagi S, Shimizu K, Takahashi M, Shirai K, Takahara A. Cardio-ankle vascular index (CAVI) differentiates pharmacological properties of vasodilators nicardipine and nitroglycerin in anesthetized rabbits. J Pharmacol Sci. 2015;128(4):185-192.
doi pubmed - Miyoshi T, Ito H, Shirai K, Horinaka S, Higaki J, Yamamura S, Saiki A, et al. Predictive value of the cardio-ankle vascular index for cardiovascular events in patients at cardiovascular risk. J Am Heart Assoc. 2021;10(16):e020103.
doi pubmed - Hitsumoto T. Clinical usefulness of the cardio-ankle vascular index as a predictor of primary cardiovascular events in patients with chronic kidney disease. J Clin Med Res. 2018;10(12):883-890.
doi pubmed - Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, et al. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461-468.
doi pubmed - Abdullah N, Blin JA, Kamalul Arifin AS, Abd Jalal N, Ismail N, Mohd Yusof NA, Abdullah MS, et al. Cardiovascular risk prediction with cardio-ankle vascular index in the malaysian cohort study. Curr Probl Cardiol. 2024;49(3):102192.
doi pubmed - Limpijankit T, Vathesatogkit P, Matchariyakul D, Yingchoncharoen T, Siriyotha S, Thakkinstian A, Sritara P. Cardio-ankle vascular index as a predictor of major adverse cardiovascular events in metabolic syndrome patients. Clin Cardiol. 2021;44(11):1628-1635.
doi pubmed - Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005;94:1-12.
doi pubmed - Hitsumoto T. Relationships between skin autofluorescence and cardio-ankle vascular index in Japanese male patients with metabolic syndrome. Cardiol Res. 2019;10(3):172-180.
doi pubmed - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419.
doi pubmed - Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73-81.
doi pubmed - Philip R, Alpert BS, Schwingshackl A, Huang X, Blakely D, Rovnaghi CR, Tran QT, et al. Inverse relationship between cardio-ankle vascular index and body mass index in healthy children. J Pediatr. 2015;167(2):361-365.e361.
doi pubmed - Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, Ohira M, et al. Inverse relationship of cardio-ankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2017;13:1-9.
- Ohashi N, Ito C, Fujikawa R, Yamamoto H, Kihara Y, Kohno N. The impact of visceral adipose tissue and high-molecular weight adiponectin on cardio-ankle vascular index in asymptomatic Japanese subjects. Metabolism. 2009;58(7):1023-1029.
doi pubmed - Nagayama D, Endo K, Ohira M, Yamaguchi T, Ban N, Kawana H, Nagumo A, et al. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes Res Clin Pract. 2013;7(2):e139-e145.
doi pubmed - Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369-381.
doi pubmed - Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, Zhao H, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):225.
doi pubmed - Hitsumoto T, Takahashi M, Iizuka T, Shirai K. Relationship between metabolic syndrome and early stage coronary atherosclerosis. J Atheroscler Thromb. 2007;14(6):294-302.
doi pubmed - Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R, Yin X, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023;14:1161521.
doi pubmed - Boutari C, DeMarsilis A, Mantzoros CS. Obesity and diabetes. Diabetes Res Clin Pract. 2023;202:110773.
doi pubmed - Sumin AN, Bezdenezhnykh NA, Bezdenezhnykh AV, Artamonova GV. Cardio-ankle vascular index in the persons with pre-diabetes and diabetes mellitus in the population sample of the russian federation. Diagnostics (Basel). 2021;11(3):474.
doi pubmed - Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293-302.
doi pubmed - Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. 2023;51(3):3000605231164548.
- Pascual-Morena C, Cavero-Redondo I, Martinez-Garcia I, Rodriguez-Gutierrez E, Luceron-Lucas-Torres M, Moreno-Herraiz N, Diaz-Goni V, et al. Exploring the influence of insulin resistance on arterial stiffness in healthy adults: from the metabolic and cardiovascular health insights of the EVasCu study. Nutrients. 2024;16(6):791.
doi pubmed - Sirivarasai J, Shantavasinkul PC, Thitiwiwatkul M, Monsuwan W, Panpunuan P, Sritara P. Association between plasma homocysteine, folate, vitamin B12 levels, and metabolic dysfunction indices in elderly with arterial stiffness. J Clin Med. 2025;14(9):2998.
doi pubmed - Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279-1289.
doi pubmed - Yokoyama H, Araki S, Kawai K, Hirao K, Oishi M, Sugimoto K, Sone H, et al. Pioglitazone treatment and cardiovascular event and death in subjects with type 2 diabetes without established cardiovascular disease (JDDM 36). Diabetes Res Clin Pract. 2015;109(3):485-492.
doi pubmed - Ohira M, Yamaguchi T, Saiki A, Ban N, Kawana H, Nagumo A, Murano T, et al. Pioglitazone improves the cardio-ankle vascular index in patients with type 2 diabetes mellitus treated with metformin. Diabetes Metab Syndr Obes. 2014;7:313-319.
doi pubmed - Kobayashi J, Saito K, Fukamachi I, Taira K, Takahashi K, Bujo H, Saito Y. Pre-heparin plasma lipoprotein lipase mass: correlation with intra-abdominal visceral fat accumulation. Horm Metab Res. 2001;33(7):412-416.
doi pubmed - Rip J, Nierman MC, Wareham NJ, Luben R, Bingham SA, Day NE, van Miert JN, et al. Serum lipoprotein lipase concentration and risk for future coronary artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2006;26(3):637-642.
doi pubmed - Hitsumoto T, Ohsawa H, Uchi T, Noike H, Kanai M, Yoshinuma M, Miyashita Y, et al. Preheparin serum lipoprotein lipase mass is negatively related to coronary atherosclerosis. Atherosclerosis. 2000;153(2):391-396.
doi pubmed - Hitsumoto T, Yoshinaga K, Aoyagi K, Sakurai T, Kanai M, Uchi T, Noike H, et al. Association between preheparin serum lipoprotein lipase mass and acute myocardial infarction in Japanese men. J Atheroscler Thromb. 2002;9(4):163-169.
doi pubmed - Moteki M, Murano T, Kurosu T, Kataoka M, Nakano M, Hiruta N, Shirai K. [Relation between cardio-ankle vascular index (Cavi) and preheparin serum lipoprotein lipase mass—effect of age adjustment]. Rinsho Byori. 2012;60(8):734-739.
pubmed - Saiki A, Takahashi Y, Nakamura S, Yamaoka S, Abe K, Tanaka S, Watanabe Y, et al. Relationship between lipoprotein lipase derived from subcutaneous adipose tissue and cardio-ankle vascular index in Japanese patients with severe obesity. Obes Facts. 2024;17(3):255-263.
doi pubmed - Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151.
doi pubmed - Yan Q, Liu S, Sun Y, Chen C, Yang S, Lin M, Long J, et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J Transl Med. 2023;21(1):519.
doi pubmed - Masugata H, Senda S, Murao K, Inukai M, Himoto T, Hosomi N, Okada H, et al. Association between urinary 8-hydroxydeoxyguanosine, an indicator of oxidative stress, and the cardio-ankle vascular index in hypertensive patients. J Atheroscler Thromb. 2012;19(8):747-755.
pubmed - Chotimol P, Saehuan C, Kumphune S. Correlation between cardio-ankle vascular index and biomarkers of oxidative stress. Scand J Clin Lab Invest. 2016;76(2):105-111.
doi pubmed - Chou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, Ferencik M. Statin use for the primary prevention of cardiovascular disease in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2022;328(8):754-771.
doi pubmed - Lee W, Kang J, Park JB, Seo WW, Lee SY, Lim WH, Jeon KH, et al. Long-term mortality and cardiovascular events of seven angiotensin receptor blockers in hypertensive patients: Analysis of a national real-world database: A retrospective cohort study. Health Sci Rep. 2023;6(2):e1056.
doi pubmed - Miyashita Y, Endo K, Saiki A, Ban N, Yamaguchi T, Kawana H, Nagayama D, et al. Effects of pitavastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, on cardio-ankle vascular index in type 2 diabetic patients. J Atheroscler Thromb. 2009;16(5):539-545.
doi pubmed - Miyashita Y, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, et al. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb. 2009;16(5):621-626.
doi pubmed - Endo K, Miyashita Y, Saiki A, Oyama T, Koide N, Ozaki H, Otsuka M, et al. Atorvastatin and pravastatin elevated pre-heparin lipoprotein lipase mass of type 2 diabetes with hypercholesterolemia. J Atheroscler Thromb. 2004;11(6):341-347.
doi pubmed - Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Miyashita Y, et al. The angiotensin II receptor antagonist valsartan enhances lipoprotein lipase mass in preheparin serum in type 2 diabetes with hypertension. Diabetes Res Clin Pract. 2006;74(3):242-248.
doi pubmed - Hitsumoto T, Takahashi M, Iizuka T, Shirai K. Effect of the angiotensin II receptor antagonist telmisartan on lipoprotein lipase mass in preheparin serum. J Atheroscler Thromb. 2008;15(3):138-145.
doi pubmed - Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060-1071.
doi pubmed - Ito R, Satoh-Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, et al. An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. J Atheroscler Thromb. 2014;21(3):248-260.
doi pubmed - Yoshida H, Ito K, Sato R, Kurosawa H, Tomono Y, Hirowatari Y, Shimizu M, et al. Clinical relevance of decreased ratios of serum eicosapentaenoic acid/arachidonic acid (AA) and docosahexaenoic acid/AA to impaired arterial stiffness. Int J Cardiol. 2014;177(2):517-519.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.