| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 10, October 2025, pages 550-555
Clinical Effects of the Concurrent Ingestion of Rosuvastatin and Magnesium Oxide: A Multicenter, Randomized, Parallel-Group Trial
Akio Nakashimaa, b, g, Kaoko Tokuraa, Momoko Misakia, Daisuke Miyazuc, Kunihisa Kobayashid, Hidenori Uratae, Hiroyuki Watanabec, Hideki Shimomuraf, Motoyasu Miyazakia, b, Osamu Imakyurea, b
aDepartment of Pharmacy, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
bDepartment of Hospital Pharmacy, Faculty of Pharmaceutical Sciences, Fukuoka University, 8-19-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
cDepartment of Pharmacy, Fukuoka Tokushukai Hospital, 4-8-25, Futsukaichi Chuo, Chikushino-shi, Fukuoka 818-0072, Japan
dDepartment of Endocrinology and Diabetes Mellitus, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
eDepartment of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
fDepartment of Cardiology, Fukuoka Tokushukai Hospital, 4-8-25, Futsukaichi Chuo, Chikushino-shi, Fukuoka 818-0072, Japan
gCorresponding Author: Akio Nakashima, Department of Pharmacy, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
Manuscript submitted August 19, 2025, accepted October 25, 2025, published online October 29, 2025
Short title: Clinical Effects of RSV With MgO
doi: https://doi.org/10.14740/jocmr6363
| Abstract | ▴Top |
Background: Cardiovascular disease (CVD) remains a leading global cause of mortality. Statins, particularly rosuvastatin (RSV), are widely used to reduce low-density lipoprotein cholesterol (LDL-C) level and prevent atherosclerotic CVD. RSV is highly liver-selective, hydrophilic, and primarily transported via organic anion transport proteins, rendering it less prone to cytochrome P450-mediated drug interactions. However, the concurrent use with magnesium oxide (MgO), a common antacid and laxative, has been shown to decrease RSV absorption by up to 50%, raising concerns regarding the clinical efficacy of RSV in patients taking MgO.
Methods: This multicenter, randomized, parallel-group comparative trial included outpatients prescribed both RSV and MgO at Fukuoka University Chikushi and Fukuoka Tokushukai Hospitals. Patients were randomly assigned to the control group with simultaneous administration or the intervention group with staggered administration of MgO at least 2 h after RSV. Data on demographics and serum levels of LDL-C, total cholesterol, high-density lipoprotein cholesterol, and triglycerides were collected at baseline and 12 weeks after study initiation. Medication adherence was monitored using a self-reported medication checklist, and patients with ≥ 80% compliance were included in the final analysis.
Results: A total of 45 patients, including 25 and 20 patients in the control and intervention groups, respectively, completed the study. The median ages were 78 and 76 years in the control and intervention groups, respectively. The baseline demographics and clinical parameters, including LDL-C, were not significantly different between the two groups. After 12 weeks, the rate of change in LDL-C level was not significantly between the control and intervention groups (P = 0.9091).
Conclusion: The concurrent use of RSV and MgO did not significantly impact the reduction in LDL-C levels compared with the staggered administration. Simplifying administration without altering efficacy can improve medication adherence, particularly in elderly patients and those with polypharmacy, by reducing treatment complexity.
Keywords: Rosuvastatin; Magnesium oxide; Low-density lipoprotein cholesterol
| Introduction | ▴Top |
According to the World Health Organization, cardiovascular diseases (CVDs) are a leading global cause of death, leading to the dedication of significant efforts to reduce CVD-associated morbidity and mortality [1]. Pharmacotherapy is a cornerstone in controlling CVD, with major guidelines established for acute coronary syndrome, including ST-elevation myocardial infarction and non-ST-elevation acute coronary syndrome, and statins are categorized as a class IA recommendation for the prevention of CVD. The American Heart Association/American College of Cardiology recommends high-dose statin therapy to achieve a 50% reduction in low-density lipoprotein cholesterol (LDL-C) levels, as a secondary approach to prevent atherosclerotic CVD in patients under 75 years of age [2].
Rosuvastatin (RSV) is a widely used statin in clinical practice for the treatment of dyslipidemia for several reasons. In addition, to its demonstrated clinical efficacy in the JUPITER [3], COSMOS [4], and APOLLO [5] trials, RSV is the only water-soluble, potent statin with strong hepatic selectivity, based on its absorption by hepatocytes via organic anion transport proteins. Additionally, unlike other potent statins, RSV is minimally metabolized by the cytochrome P450 enzymes CYP3A4 and CYP2C19, suggesting a lower risk of drug-drug interactions in patients treated with RSV [6]. However, a unique feature of RSV is its interaction with magnesium oxide (MgO), wherein concurrent ingestion can reduce RSV absorption by up to 50% [7].
MgO is widely used as a laxative and antacid in East Asia [8]. The increasing prevalence of chronic constipation among elderly patients in aging societies has resulted in an increase in MgO use [9], with a consequent increase in the likelihood of concurrent ingestion of RSV and MgO in clinical practice, especially in patients receiving treatment for lifestyle-related diseases.
However, no prospective study to date has investigated the clinical effects of the concurrent administration of RSV and MgO. Therefore, we aimed to evaluate the clinical impact of the concurrent administration of RSV and MgO on LDL-C levels in a multicenter, randomized, parallel-group comparative trial.
| Materials and Methods | ▴Top |
Study design and participants
This multicenter, randomized, parallel-group comparative trial was conducted in Fukuoka University Chikushi and Fukuoka Tokushukai Hospitals between March 1, 2017 and March 1, 2021. Outpatients concurrently prescribed RSV and MgO were eligible, whereas those unable to provide informed consent, those unable to manage their own medication, those taking preparations containing both magnesium and aluminum, and those otherwise deemed inappropriate for eligibility by the study investigators were excluded. All participants were Japanese.
Eligible patients were randomized using a block randomization method (block size = 4) without stratification into two groups: 1) control group: concurrent ingestion of RSV and MgO; 2) intervention group: staggered ingestion of MgO at least 2 h after RSV.
The study was approved by the Ethics Committee of the Fukuoka University School of Medicine (No. R16-048). It complied with the ethical standards of the responsible institution on human subjects as well as with the Declaration of Helsinki.
Indications and medication details
RSV was prescribed for dyslipidemia management in both primary and secondary prevention of cardiovascular disease, based on clinical indication.
MgO was prescribed for the management of chronic constipation in all participants. Both medications were taken daily.
Participants were instructed to take RSV and MgO after meals, with no difference in administration timing between groups.
Data collection
Demographic and clinical characteristics at baseline and 12 weeks after study initiation were collected using electronic medical records and included data on age, sex, body weight, body mass index, adherence rate, RSV and MgO dosages, and clinical laboratory values (total cholesterol (T-Cho), high-density lipoprotein cholesterol (HDL-C), LDL-C, and triglycerides (TG)).
Patients receiving other lipid-lowering drugs (e.g., bile acid sequestrants, fibrates) or aluminum-containing antacids were excluded.
Medication possession ratio
Medication adherence was evaluated using a self-reported medication checklist, and patients with a medication adherence rate of ≥ 80% were included in the final analysis. The checklist was distributed at study initiation and collected after the 12-week study period. Patients marked the checklist each time they took the medications as prescribed (concurrent ingestion of both medications or the staggered ingesting of medications with a minimum of 2-h interval). The medication possession ratio was calculated as follows: medication possession ratio = (100 × number of checks/number of prescription days). Although this method is practical, we acknowledge that self-reported checklists may introduce bias compared with more objective measures such as pill counts or electronic monitoring.
Statistical analysis
The baseline patient characteristics were compared between the control and intervention groups. In addition, changes in blood tests between baseline and 12 weeks were compared. The primary endpoint was the percent changes in LDL-C levels after 12 weeks of intervention. Categorical and continuous variables were analyzed using Fisher’s exact and the Mann-Whitney U tests, respectively. Statistical significance was defined at a P value of < 0.05. As this was an exploratory pilot study, no formal power calculation was performed prior to initiation. All statistical analyses were conducted using JMP® Pro 17.2.0 (SAS Institute, Cary, NC, USA).
| Results | ▴Top |
Patient characteristics
After excluding patients who met the exclusion criteria or dropped out during the study period, 25 patients with a median age of 78 years were assigned to the control group and 20 patients with a median age of 76 years were assigned to the intervention group (Fig. 1). Table 1 shows the comparison of the baseline characteristics between the two study groups. Briefly, age, sex, body mass index, RSV and MgO dosages, and clinical laboratory values (T-Cho, HDL-C, LDL-C, and TG) were not significantly different between the two groups at baseline.
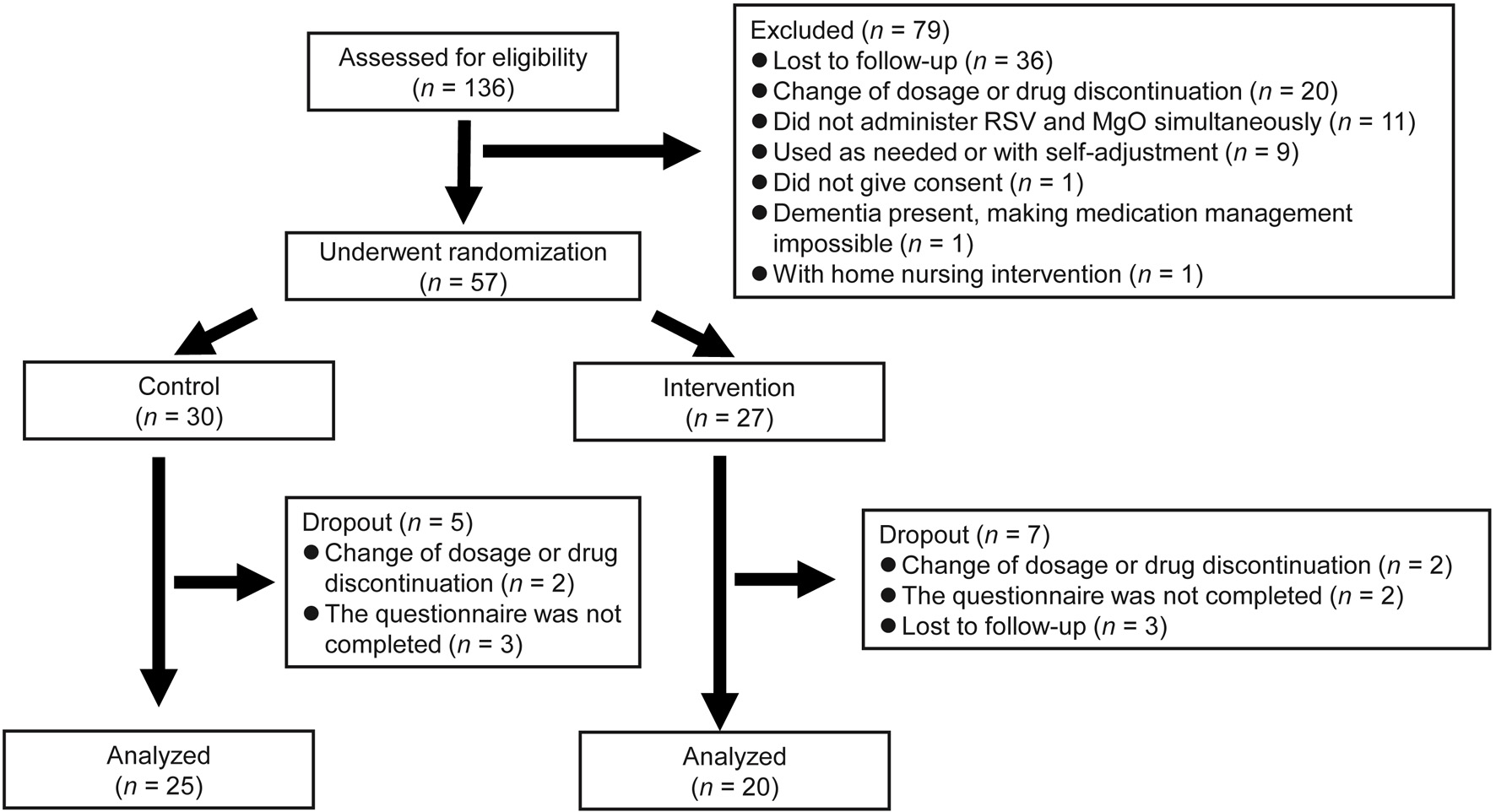 Click for large image | Figure 1. Study flowchart. |
 Click to view | Table 1. Baseline Characteristics of Patients in the Control and Intervention Groups |
Comparison of changes in T-Cho, HDL-C, LDL-C, and TG levels
Figure 2 shows the percent changes in T-Cho, HDL-C, LDL-C, and TG levels after 12 weeks of intervention. The median percent change in T-Cho levels was -0.0275% (interquartile range (IQR), -0.1759% to 0.083673%) in the intervention group and -0.00568% (IQR, -0.08459% to 0.042129%) in the control group, without a statistically significant difference between the two groups (P = 0.7062). HDL-C levels were -0.05742% (IQR, -0.10033% to 0.167872%) in the intervention group and -0.00806% (IQR, -0.06667% to 0.068829%) in the control group, without a statistically significant difference between the two groups (P = 0.6749). The median percent change in LDL-C levels was -0.0258% (IQR, -0.12963% to 0.092614%) in the intervention group and 0.028846% (IQR, -0.17602% to 0.113636%) in the control group, without a statistically significant difference between the two groups (P = 0.9091). TG levels were 0.073718% (IQR, -0.34795% to 0.43816%) in the intervention group and 0.034678% (IQR, -0.17455% to 0.42576%) in the control group, without a statistically significant difference between the two groups (P = 0.8588).
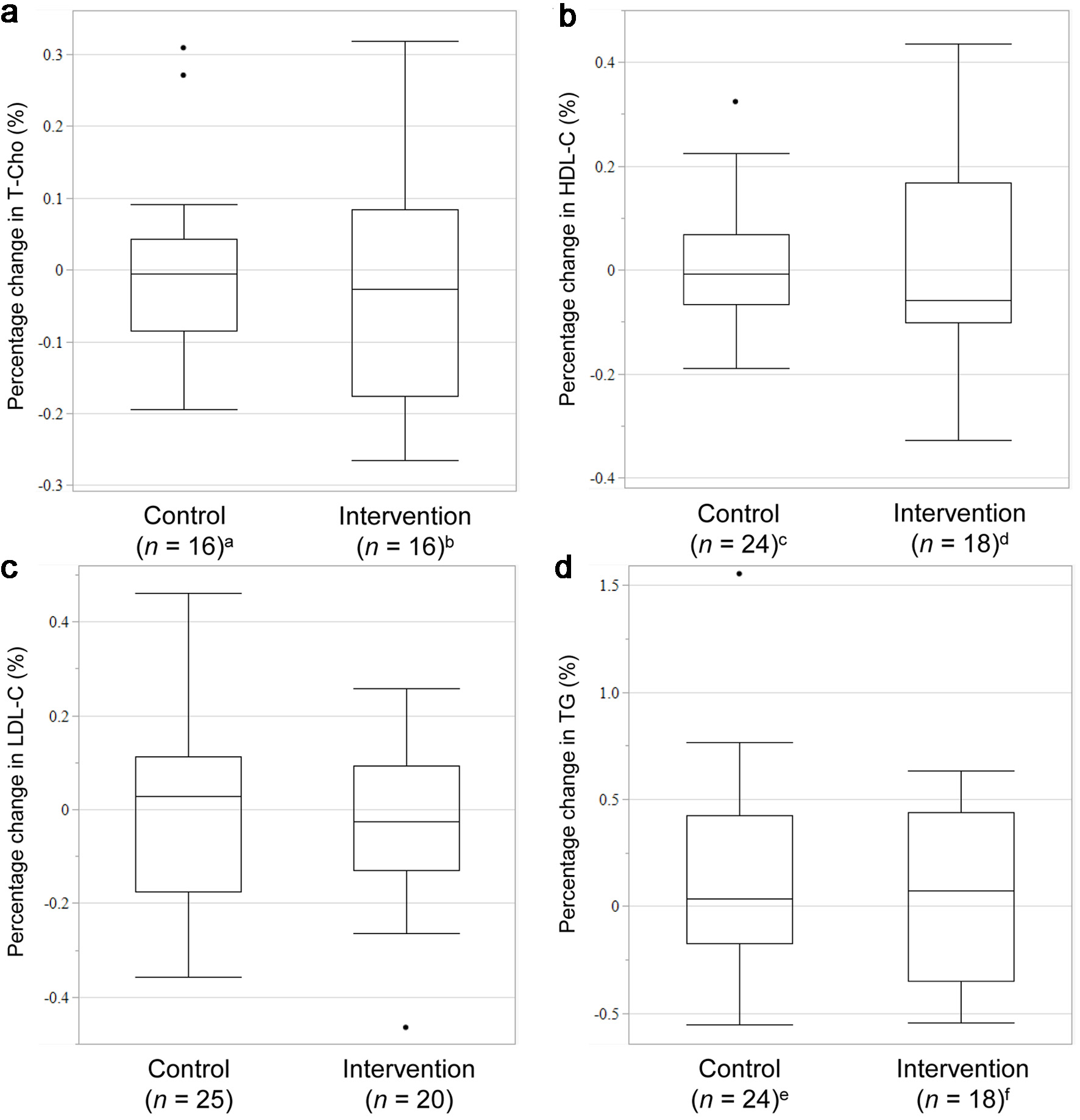 Click for large image | Figure 2. Rate of change in the levels of T-Cho (a), HDL-C (b), LDL-C (c), and TG (d). Missing data: an = 9, bn = 4, cn = 1, dn = 2, en = 1, fn = 2. T-Cho: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides. |
| Discussion | ▴Top |
In this first prospective study investigating the clinical impact of the concomitant use of RSV and MgO, we prospectively evaluated the changes in lipid-related clinical laboratory values in patients ingesting RSV and MgO simultaneously or in a staggered schedule. As shown in Figure 2, the rate of change in LDL-C levels was not significantly different between the control and intervention groups, indicating that concurrent RSV and MgO administration might have a minimal impact on the rate of change in LDL-C levels.
Martin et al [7] reported that delaying antacid administration by 2 h reduced its impact on plasma RSV concentrations in Western cohorts, contradicting our findings, which might be attributed to racial differences in the pharmacokinetics. According to Lee et al [10], steady-state plasma RSV concentrations in Japanese participants are approximately twofold higher than those in White participants, indicating that plasma RSV concentrations were higher in Japanese participants. Furthermore, although RSV is transported into hepatocytes by the organic anion transporting polypeptide 1B1, encoded by SLCO1B1, the polymorphisms common in East Asian populations primarily affect hepatic uptake after absorption rather than gastrointestinal absorption. These polymorphisms have been reported to cause increased blood concentrations of RSV and its active metabolite, N-desmethyl RSV [11]. Therefore, while SLCO1B1 variants may contribute to higher systemic exposure to RSV in Japanese patients, they are unlikely to directly influence the physicochemical interaction with MgO in the gut. The presence of these polymorphisms might, however, partially explain the modest interindividual variability in LDL-C response observed in this cohort.
The interaction between RSV and MgO is thought to be primarily physicochemical. MgO raises gastric pH and can form poorly soluble complexes with the anionic RSV molecule, thereby reducing its intestinal absorption. However, the magnitude of this effect depends on several factors, including the timing of administration, gastric emptying rate, and dietary status. Kawakami et al [12] reported that postprandial administration mitigates such interactions by stabilizing gastric conditions. In our study, all participants took both RSV and MgO after meals, when food likely buffered gastric pH changes and slowed gastric emptying. Food intake also stimulates bile acid secretion, which enhances the solubility and intestinal absorption of hydrophilic statins such as RSV. These postprandial conditions may have minimized the impact of MgO on RSV absorption.
Staggering administration increases the dosing frequency and complicates prescriptions, which can reduce adherence [13]. Although the inhibition of absorption has been reported in patients taking RSV and MgO, adjustments to the timing of administration might not be necessary for Japanese patients.
Study limitations
Several limitations should be noted.
First, this was a pilot study without formal power analysis, and the relatively small sample size may have limited statistical power, increasing the possibility of type II error. Second, medication adherence was evaluated using a self-reported checklist, which may have introduced reporting bias. Third, compliance with the 2-h dosing interval in the intervention group could not be objectively verified. Fourth, lifestyle factors such as diet and exercise, as well as comorbidities were not controlled and may have influenced lipid metabolism. Fifth, all participants were Japanese, which may limit the generalizability of the findings to other ethnic populations with different genetic and dietary backgrounds. Finally, the duration of prior exposure to RSV and MgO before enrollment was not assessed, and previous dose adjustments may have influenced baseline lipid levels.
Despite these limitations, our findings provide preliminary clinical evidence that concurrent RSV and MgO administration is safe and pharmacologically acceptable in elderly Japanese patients receiving long-term statin therapy.
Conclusion
In conclusion, the concurrent administration of RSV and MgO did not significantly affect LDL-C reduction compared with staggered administration. Simplifying dosing schedules without compromising therapeutic efficacy may improve medication adherence and reduce treatment complexity, particularly among elderly patients with multiple medications. Further large-scale, controlled studies with pharmacokinetic assessments and genotyping for SLCO1B1 variants are warranted to confirm these results and better elucidate the clinical and mechanistic implications of the MgO-RSV interaction.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Not applicable.
Author Contributions
AN and OI contributed to the concept and design of the study; AN, KT, MM, and DM conducted the study; AN and MM were involved in the data analysis and interpretation of the results; AN drafted the manuscript; KK, HU, HW, HS, and OI supervised the entire project and reviewed the manuscript; all authors have read and approved the final version of the manuscript.
Data Availability
All data generated or analyzed during the study are included in this published article.
Abbreviations
CVD: cardiovascular disease; HDL-C: high-density lipoprotein cholesterol; IQR: interquartile range; LDL-C: low-density lipoprotein cholesterol; MgO: magnesium oxide; T-Cho: total cholesterol; TG: triglycerides
| References | ▴Top |
- Luo Y, Liu J, Zeng J, Pan H. Global burden of cardiovascular diseases attributed to low physical activity: An analysis of 204 countries and territories between 1990 and 2019. Am J Prev Cardiol. 2024;17:100633.
doi pubmed - Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1082-e1143.
doi pubmed - Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207.
doi pubmed - Takayama T, Hiro T, Yamagishi M, Daida H, Hirayama A, Saito S, Yamaguchi T, et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J. 2009;73(11):2110-2117.
doi pubmed - Amemiya K, Yokoi H, Domei T, Shirai S, Ando K, Goya M, Iwabuchi M. Suppressive effects of standard-dose rosuvastatin therapy on the progression of coronary atherosclerosis in Japanese patients: the APOLLO study. J Atheroscler Thromb. 2014;21(12):1298-1307.
doi pubmed - Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41(5):343-370.
doi pubmed - Martin PD, Schneck DW, Dane AL, Warwick MJ. The effect of a combination antacid preparation containing aluminium hydroxide and magnesium hydroxide on rosuvastatin pharmacokinetics. Curr Med Res Opin. 2008;24(4):1231-1235.
doi pubmed - Mori H, Tack J, Suzuki H. Magnesium oxide in constipation. Nutrients. 2021;13(2).
doi pubmed - Kang SJ, Cho YS, Lee TH, Kim SE, Ryu HS, Kim JW, Park SY, et al. Medical management of constipation in elderly patients: systematic review. J Neurogastroenterol Motil. 2021;27(4):495-512.
doi pubmed - Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78(4):330-341.
doi pubmed - Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, Kim K, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71(3):329-340.
doi pubmed - Kawakami J, Matsuse T, Kotaki H, Seino T, Fukuchi Y, Orimo H, Sawada Y, et al. The effect of food on the interaction of ofloxacin with sucralfate in healthy volunteers. Eur J Clin Pharmacol. 1994;47(1):67-69.
doi pubmed - Pantuzza LL, Ceccato M, Silveira MR, Junqueira LMR, Reis AMM. Association between medication regimen complexity and pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol. 2017;73(11):1475-1489.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.