| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 10, October 2025, pages 556-564
Optimal Timing of Post-Alteplase Brain Computed Tomography: Routine Twenty-Four-Hour Versus Extended Forty-Eight-Hour for Detecting Asymptomatic Intracranial Hemorrhage
Pranisa Botkosaa , Thanin Lokeskraweea, f
, Donsuk Pongnikornb
, Natthaphon Pruksathorna
, Suppachai Lawanaskolc
, Jayanton Patumanondd, Jarupa Yaowalaornga
, Suwapim Chanlaore, Wanwisa Bumrungpagdeee, Chawalit Lakdeee
aDepartment of Emergency Medicine, Lampang Hospital, Lampang, Thailand
bChiangmai Neurological Hospital, Chiang Mai, Thailand
cChaiprakarn Hospital, Chiang Mai, Thailand
dClinical Epidemiology and Clinical Statistics Unit, Faculty of Medicine, Naresuan University, Phitsanulok, Thailand
eDepartment of Radiology, Buddhachinaraj Phitsanulok Hospital, Phitsanulok, Thailand
fCorresponding Author: Thanin Lokeskrawee, Department of Emergency Medicine, Lampang Hospital, Lampang, Thailand
Manuscript submitted September 7, 2025, accepted October 13, 2025, published online October 29, 2025
Short title: Post-Alteplase CT Timing for Asymptomatic ICH
doi: https://doi.org/10.14740/jocmr6378
| Abstract | ▴Top |
Background: Intracranial hemorrhage (ICH) is a major complication of intravenous alteplase for acute ischemic stroke. Asymptomatic ICH (asICH) does not cause immediate neurological decline but may have medicolegal consequences if undetected. In Asian populations, up to 24% of post-alteplase ICH occurs beyond 24 h, suggesting that routine computed tomography (CT) at 24 h may underestimate its incidence. This study evaluated the diagnostic yield of CT at 24 h versus 48 h to determine the optimal timing for ICH detection.
Methods: A retrospective cohort study was conducted at Lampang Hospital from March 2017 to December 2024. Patient outcomes were analyzed using an illness-death multistate model with three health states: no ICH, asICH, and symptomatic ICH (sICH). Three transitions were modeled - from no ICH to asICH, from no ICH to sICH, and from asICH to sICH - assuming irreversible progression. The proportions of asICH detected by CT at 24 h versus 48 h were compared using the exact probability test.
Results: After exclusions, a total of 555 patients were eligible (54.8% male, mean age 65 years). Final transition outcomes identified 455 patients with no ICH, 51 with asICH, and 49 with sICH. Brain CT at 24 h detected 30.2% of asICH cases, while 48-h imaging detected 96.8%, with a significant improvement (P < 0.001).
Conclusions: CT imaging at 48 h post-alteplase improves asICH detection compared with the 24-h scan, reducing missed cases without added radiation or resource burden. Multicenter validation is needed to confirm whether 48 h should replace the current standard.
Keywords: Ischemic stroke; Thrombolytic therapy; Tissue plasminogen activator; Tomography, X-ray computed; Intracranial hemorrhages; Time factors
| Introduction | ▴Top |
After receiving intravenous (IV) thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA) for acute ischemic stroke (AIS), patients are at risk of a major complication - intracranial hemorrhage (ICH) - commonly referred to as post-alteplase ICH [1, 2]. This complication can follow one of three clinical courses: 1) no ICH; 2) asymptomatic ICH (asICH); or 3) symptomatic ICH (sICH). The incidence of post-alteplase ICH, encompassing both asICH and sICH, varies across studies due to differences in populations, definitions, and clinical practices. Reported rates range from 6.4% to 27% for overall ICH and 1.9% to 8.7% for sICH [2-9].
Following alteplase administration, patients undergo close monitoring of vital signs and neurological status, along with a routine brain computed tomography (CT) scan performed at approximately 24 h, as recommended by the American Heart Association (AHA) 2019 [10]. In those with mild symptoms or low risk, the scan usually reveals no ICH. However, some patients with initially negative findings who later develop subtle neurological changes may require repeated imaging - up to one to three scans - to detect ICH (local data from Lampang Hospital). Furthermore, in 2023, 76% of post-alteplase ICH cases were detected within this initial 24-h window, while 24% represented delayed bleeding beyond that period [11].
Previous studies focused solely on sICH, particularly cases resulting in neurological surgery and morbidity. In contrast, asICH may not lead to significant clinical consequences; however, missed diagnoses can raise medicolegal concerns. It is therefore important to balance cost-effectiveness: overuse of brain CT increases radiation exposure, whereas a parsimonious approach with higher imaging thresholds may result in missed diagnoses. Notably, no previous studies have applied time-to-event analysis or evaluated asICH through a comparative scenario. This study uses real-world data to simulate two strategies: routine brain CT at 24 h versus 48 h. If the 48-h approach can effectively detect ICH while reducing the number of scans, it may support revisions to current clinical practice guidelines.
| Materials and Methods | ▴Top |
Study design
This retrospective observational cohort study was conducted at the Emergency Department (ED) and Stroke Unit of Lampang Hospital, a tertiary regional stroke center in Northern Thailand, with an estimated 2,000 acute stroke cases annually, comprising 1,400 ischemic and 600 hemorrhagic cases (a 70:30 ratio). Approximately 80 patients receive IV alteplase each year. The study period was from March 2017 to December 2024. Eligible participants were AIS patients who received IV alteplase without contraindications. Data collected included the date and time of alteplase administration, as well as the date and time of follow-up brain CT scans, with intervals calculated in minutes and converted to hours.
Participants and data collection
We included patients aged 18 years or older, who presented with acute stroke symptoms and had a National Institutes of Health Stroke Scale (NIHSS) score ≥ 4. All patients arrived at the ED within 3 to 4.5 h, underwent an initial brain CT scan showing no evidence of ICH, and then received IV alteplase. Exclusion criteria included patients who did not receive the full alteplase dose, such as due to minor bleeding during administration; those who underwent mechanical thrombectomy (MT) to avoid confounding from combined interventions; and those with a final diagnosis classified as the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) subtype 4 (other determined etiology) or subtype 5 (undetermined etiology) [12], due to their distinct pathophysiological mechanisms of bleeding that differ from those typically observed in thrombolysis-related ICH.
Baseline characteristics including sex, age, NIHSS score, actual body weight, number of brain CT scans during admission, and in-hospital mortality were collected.
In the context of acute stroke management requiring timely alteplase administration, dosing is calculated based on the patient’s actual body weight. Measurement of height for body mass index (BMI) calculation is not routinely performed in the stroke fast-track process, as it would prolong both door-to-needle and onset-to-needle times.
Endpoints
The primary endpoint was the detection of asICH on follow-up brain CT scans performed at the routine 24-h versus the extended 48-h time point after IV alteplase administration. The timing of sICH detection was not considered an outcome of interest, as patients with clinical deterioration always underwent immediate brain CT imaging, whereas asICH was more likely to be over-investigated and thus was defined as the primary outcome.
Definitions of post-alteplase ICH
Adapted from previous studies [1, 2], post-alteplase ICH was classified into three categories - no ICH, asICH, and sICH. In this study, patients were classified according to their final transitioned outcome within 7 days after alteplase administration: 1) No ICH: patients with no evidence of hemorrhage on follow-up brain CT scans during the 7-day observation period. This included patients who underwent multiple follow-up scans without hemorrhage and had no unscheduled ED revisit, while attending scheduled Outpatient Department (OPD) follow-up, as verified in the medical record; 2) asICH: patients with ICH detected on a brain CT scan without any associated clinical deterioration. This included patients whose earlier follow-up brain CT scans showed no ICH but who later developed it, provided they remained clinically stable without neurological worsening throughout the 7-day observation period; 3) sICH: patients with ICH accompanied by clinical deterioration, defined as an increase in the NIHSS score of ≥ 4 points, where the hemorrhage was judged to be the most likely cause of deterioration. When edema and hemorrhage coexisted and causality was uncertain, the deterioration was adjudicated as being attributed to hemorrhage. Patients could transition from no ICH or asICH on earlier follow-up brain CT scans to sICH during the 7-day period; in such cases, the final transitioned status of sICH was assigned; 4) Time to first detection of asICH: interval from IV alteplase bolus to the time when asICH was first detected; 5) First follow-up brain CT scan findings: the status of brain CT scans (no ICH, asICH, or sICH) observed at the time of the first follow-up scan after IV alteplase administration; 6) Final transitioned outcomes within 7 days: the last recorded status of brain CT scan findings (no ICH, asICH, or sICH) during the entire 7-day follow-up period. This reflects the ultimate transition state and may differ from the initial findings at the first follow-up scan; 7) Grouping of 24-h and 48-h scenarios: based on the multi-state illness-death model (counterfactual modeling, not real-time clinical scheduling), patients were grouped by the modeled timing of asICH detection. If the model estimated asICH detection ≤ 24 h after alteplase, patients were assigned to the 24-h scenario; if detection occurred > 24 h (i.e., the extended strategy), patients were assigned to the 48-h scenario.
Classification notes
Patients with clinical deterioration and a follow-up brain CT scan showing only cerebral edema (without hemorrhage) were classified as no ICH; those with edema and minor hemorrhage, without significant clinical worsening, were classified as asICH.
Adjudication
To enhance reliability and reduce bias, final transitioned outcomes were independently assessed by two of three blinded experts (an emergency physician, an internist, and a radiologist), with majority rule for disagreements.
Determinant
The determinant was the modeled timing of asICH detection - 24-h versus 48-h scenarios.
Confounders
Confounding was not applicable to this analysis, as the study aimed solely to describe the overall timing of post-alteplase ICH under two modeled scenarios - 24-h versus 48-h -without attempting to establish causal relationships.
Study size estimation
The sample size was calculated from a pilot study of 50 patients. Because the study included three outcome groups - no ICH, asICH, and sICH - the estimation was based on pairwise comparisons using a two-sided test with a 5% significance level and 80% power.
No ICH vs. sICH
The expected prevalence of sICH on brain CT scans at 48 h was 8.0%, compared with 0% in the reference group. Because Stata does not allow calculations with an absolute zero, a near-zero value (0.00000001%) was substituted. With a 16:1 allocation ratio, the required sample size was 33 sICH and 528 no ICH cases.
No ICH vs. asICH
The expected prevalence of asICH was 10.7% versus 0%. With a 10:1 allocation ratio, the required sample size was 28 asICH and 280 no ICH cases.
To ensure adequate power across all comparisons, the largest required sample sizes were adopted: 33 for sICH, 28 for asICH, and 528 for no ICH, giving a total required sample size of 589 participants.
Statistical analysis
Baseline characteristics were compared across the three final transitioned outcome groups - no ICH, asICH, and sICH - using a non-parametric test for trend. Time-to-event analysis was performed using a multistate illness-death model [13] to account for transitions among three clinical states: no ICH (state 1), asICH (state 2), and sICH (state 3). The maximum duration of follow-up brain CT scans was 7 days, consistent with our predefined protocol. Three transitions were defined: 1) Transition 1 (no ICH → asICH): time to the first detection of asICH, regardless of whether patients subsequently progressed to sICH; 2) Transition 2 (no ICH → sICH): time to the first detection of sICH in patients who developed it directly from the no ICH state; 3) Transition 3 (asICH → sICH): time to the first detection of sICH in patients who had initially developed asICH.
Patients with no evidence of ICH on all follow-up brain CT scans were classified as having no transition (Fig. 1).
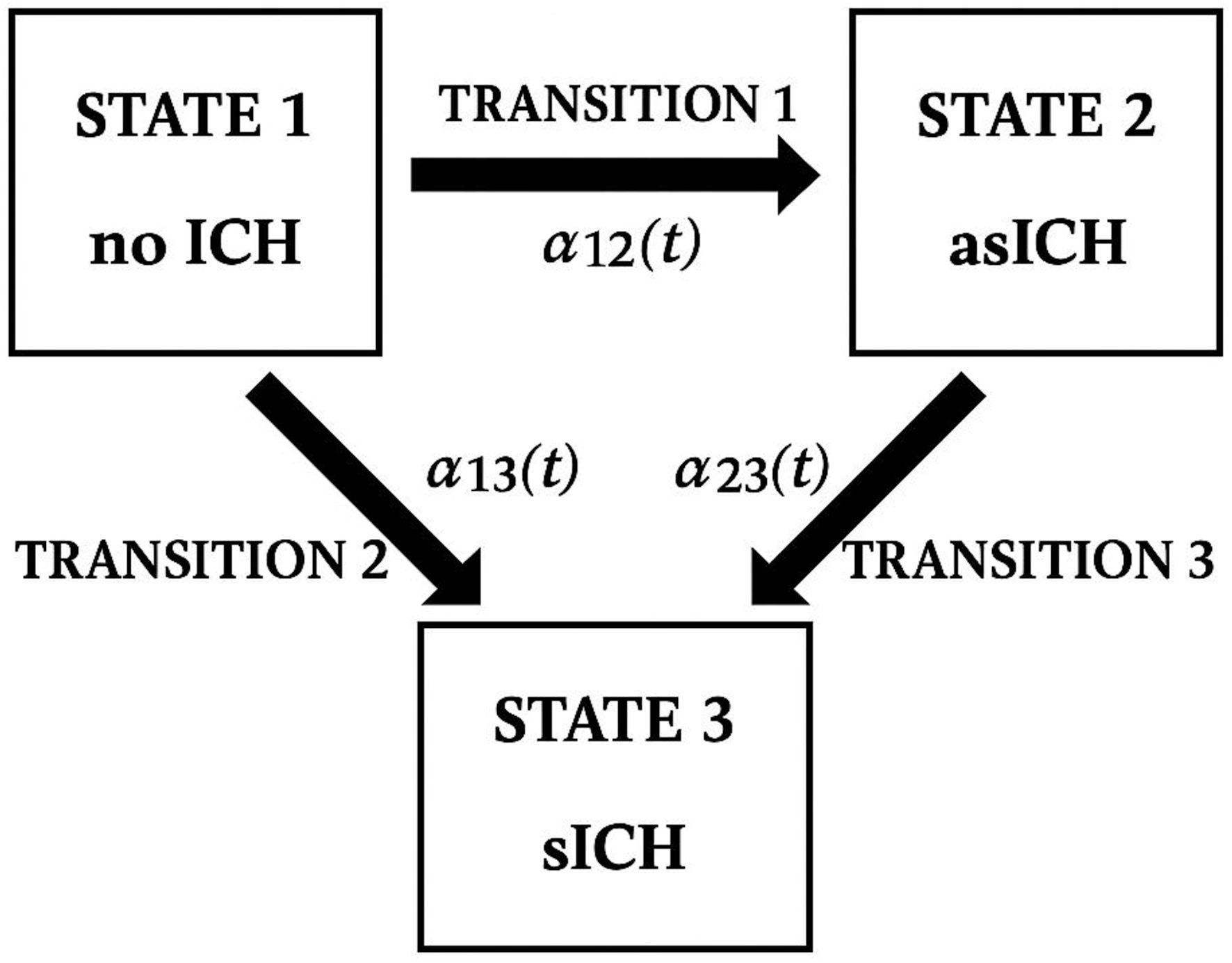 Click for large image | Figure 1. Conceptual illustration of post-alteplase outcomes and transitions adapted from a multi-state (illness-death) model. ICH: intracranial hemorrhage; asICH: asymptomatic intracranial hemorrhage; sICH: symptomatic intracranial hemorrhage. |
Cumulative incidence functions were estimated using flexible parametric survival models based on the Royston-Parmar approach, implemented via the merlin package in Stata version 18.0 (StataCorp, College Station, TX, USA; licensed).
Given the dynamic nature of these transitions over time, a multistate survival analysis using the illness-death model was employed to evaluate progression patterns and the probabilities of each outcome.
The study protocol was registered in the Thai Clinical Trials Registry (TCTR) (TCTR20250828002). The Institutional Review Board of Lampang Hospital approved the study protocol (CERT NO. 071/68). The study was conducted in accordance with the ethical standards of the responsible institutional committee on human subjects and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
| Results | ▴Top |
Between March 2017 and December 2024, a total of 555 patients remained eligible for analysis after exclusions. The numbers presented in the study flow diagram reflect outcomes based on the first follow-up brain CT scan. Subsequent transitions - such as progression from no ICH to asICH, asICH to sICH, or directly from no ICH to sICH - were not counted in the flowchart, as patient outcomes evolved over time and were analyzed using a multistate illness-death model. This model allowed us to capture dynamic transitions without redundantly counting individuals at multiple outcome stages (Fig. 2).
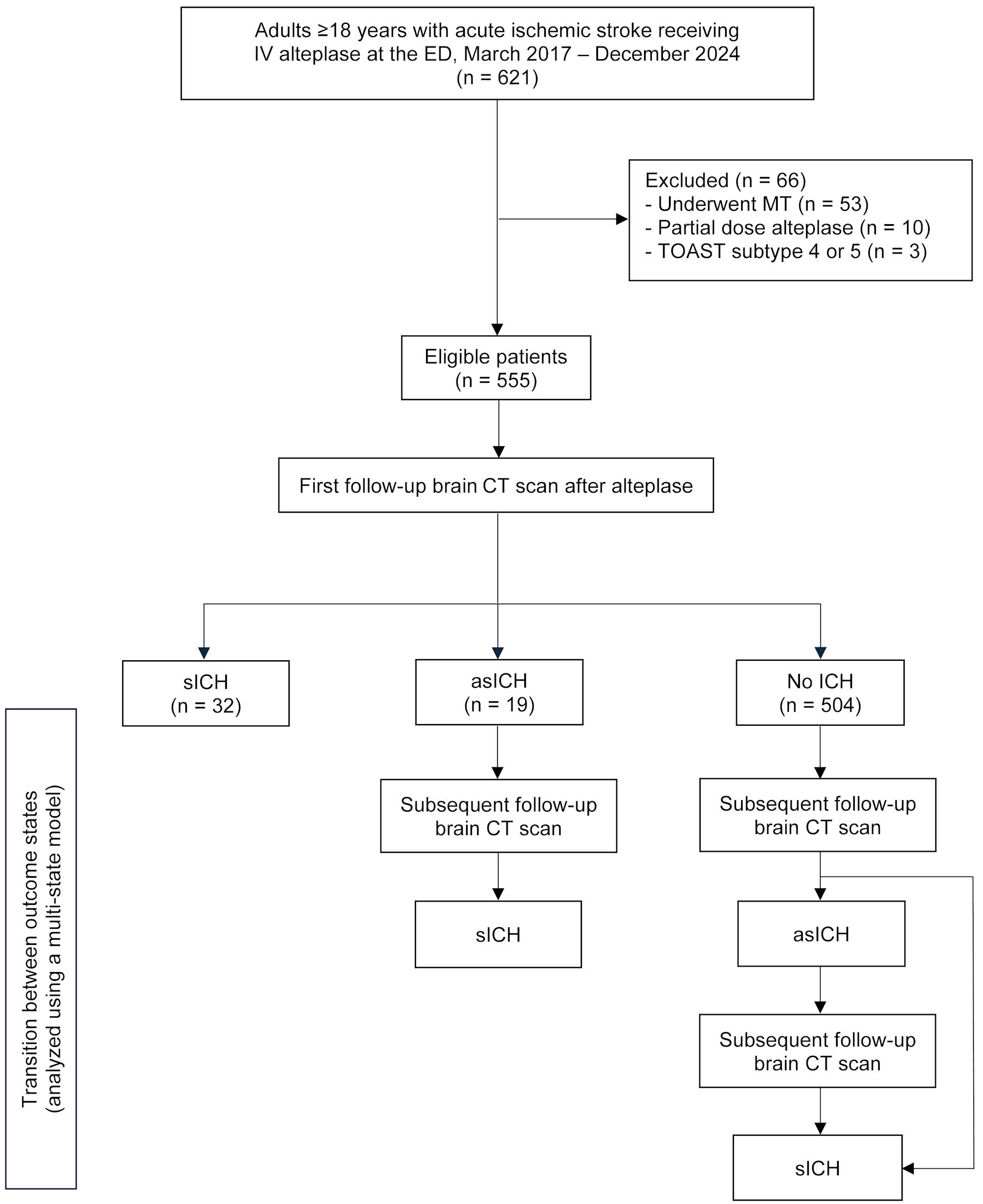 Click for large image | Figure 2. Study flow diagram. Numbers are provided only for groups identified after the first follow-up brain CT scan; subsequent counts are omitted because outcomes evolved dynamically over time and were analyzed using a multi-state (illness-death) model. IV: intravenous; ED: emergency department; MT: mechanical thrombectomy; TOAST: Trial of Org 10172 in Acute Stroke Treatment; CT: computed tomography; ICH: intracranial hemorrhage; asICH: asymptomatic intracranial hemorrhage; sICH: symptomatic intracranial hemorrhage. |
Based on the final outcomes at 7-day follow-up, 455 patients had no ICH, 51 were classified as asICH, and 49 as sICH. The majority were male (54.8%), with a mean age of 65 years. Patients with sICH had higher initial NIHSS scores, underwent more follow-up brain CT scans, and experienced the highest mortality. Notably, mortality in the no ICH group exceeded that of the asICH group, largely attributable to neurological causes such as brain edema and to non-neurological causes such as subsequent nosocomial infections (Table 1).
 Click to view | Table 1. Baseline Characteristics by Final Transitioned Outcomes at 7-Day Follow-Up |
Among the 51 patients classified as asICH at the final 7-day outcome, up to 30% underwent brain CT scans more than once, and nearly 10% had as many as three - despite no transition beyond this stage. This suggests potential overuse of imaging, as these patients ultimately remained in the same non-severe category without progressing to a more serious condition (Table 2).
 Click to view | Table 2. Frequency of Brain CT Scans for Asymptomatic Intracranial Hemorrhage Within 7 Days |
The number of asICH cases shown in the study flow diagram (n = 19) reflects only those identified on the first static follow-up brain CT scan. However, when analyzed using the multistate illness-death model, a total of 63 patients were found to have experienced a first transition to asICH, including those who were initially classified as no ICH. The routine 24-h brain CT scan detected only 19 of these 63 cases (30.2%), whereas a 48-h imaging scenario would have identified 61 cases (96.8%), demonstrating a significantly improved detection rate (P < 0.001) (Table 3).
 Click to view | Table 3. Comparison of First Asymptomatic Intracranial Hemorrhage (asICH) Detection Between 24-Hour Routine and 48-Hour Extended Brain Computed Tomography Scenarios |
Cumulative incidence curves of transitions to asICH (blue) and sICH (red) over time were derived from a multistate illness-death model. The x-axis represents hours since alteplase administration (up to 7 days), while the y-axis shows model-estimated probabilities of transition. These are smoothed estimates based on a flexible parametric survival model (Royston-Parmar via Stata’s merlin), rather than direct detection rates. The sharp rise and early plateau of the blue curve around 48 h indicate that most asICH transitions occurred within the first 48 h. In contrast, the red sICH curve shows a more gradual increase, reflecting later detection linked to clinical deterioration. The curves illustrate transition timing and patterns - not actual proportions - so quantitative interpretation of the y-axis is discouraged (Fig. 3).
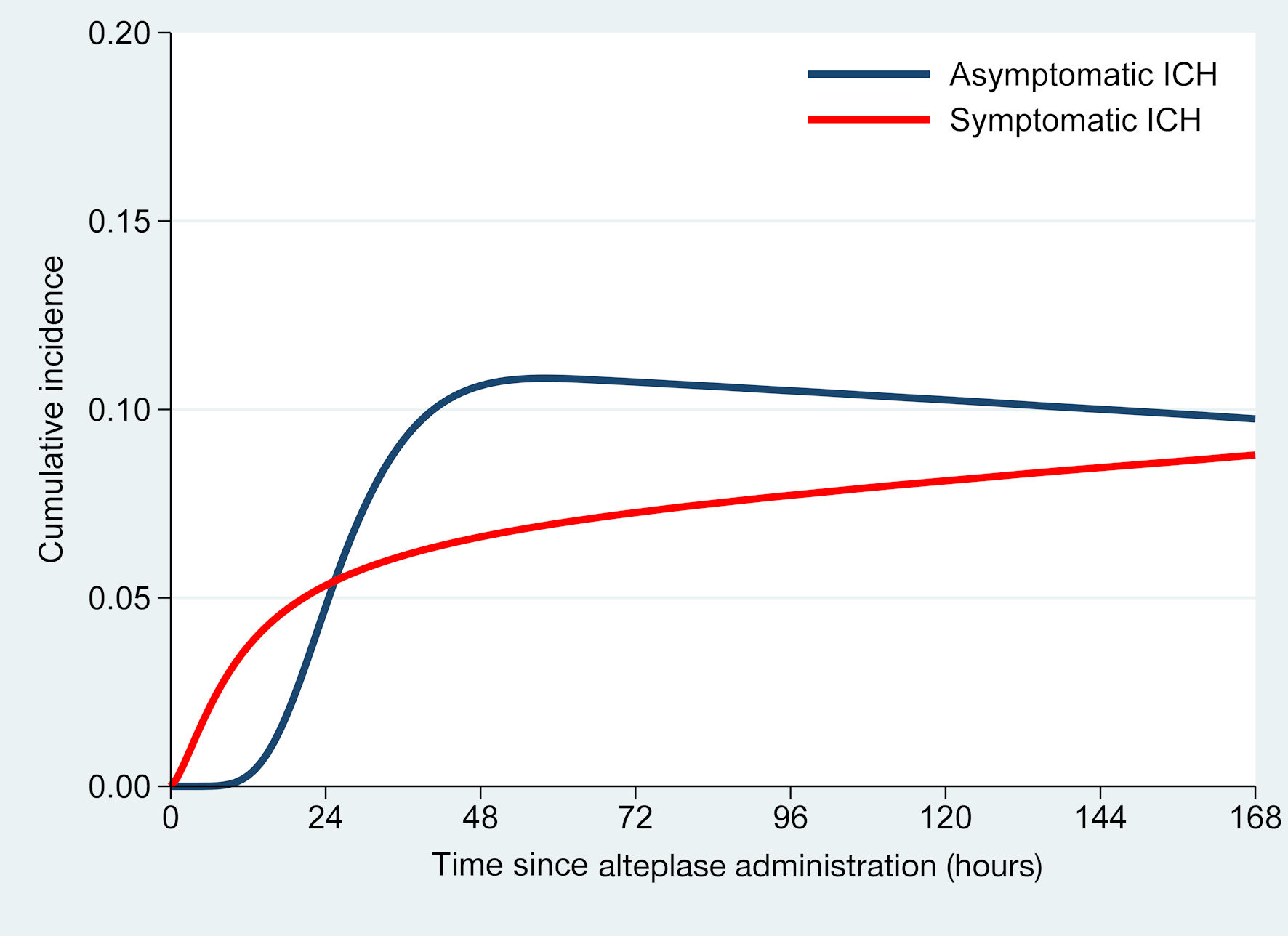 Click for large image | Figure 3. Cumulative incidence of intracranial hemorrhage (ICH) over time following intravenous alteplase administration. |
| Discussion | ▴Top |
This study demonstrated that extending the follow-up brain CT from 24 h to 48 h after IV alteplase administration markedly improved the detection of asICH, increasing the sensitivity from 30.2% to 96.8%. Using a multistate illness-death model, we found that most asICH transitions occurred within the first 48 h, whereas symptomatic cases developed later and were associated with clinical deterioration. These findings indicate that the conventional 24-h imaging protocol may underestimate delayed bleeding events. Extending the follow-up scan to 48 h could enhance post-thrombolysis safety monitoring without increasing radiation exposure or imposing additional resource burden.
Most existing studies have concentrated on sICH because of its association with severe outcomes and neurosurgical intervention, whereas asICH has been largely neglected in the literature and is seldom reported. In Thailand, patients with minor post-discharge symptoms often present to private hospitals, and even when asymptomatic hemorrhagic infarction (HI) type 1 or 2 is identified, conservative treatment remains standard. Nevertheless, in the public healthcare system, delayed or missed detection of asICH can lead to medicolegal concerns, as families may view this as a basis for legal action against government hospitals.
Post-alteplase ICH may occur not only within 24 h but also up to 36 h by the mSITS-MOST criteria [5] and as late as 7 days in the ECASS II and III trials [2, 14]. Incidences beyond 24 h have been reported in 3-18% of cases [15-22]. In Asian populations, delayed hemorrhage is disproportionately more frequent, with up to one quarter occurring after 24 h [3, 11]. This phenomenon, often referred to as the “East Asian paradox,” has been attributed to differences in coagulation and platelet function, including lower procoagulant factor levels, reduced protein C pathway activity, and greater vulnerability to hemorrhage - factors that collectively predispose to both early and delayed bleeding [22-24].
A key consideration is that follow-up CT scans after IV alteplase were not performed at exactly 24 h. An asICH detected beyond this point may have occurred earlier but remained undiagnosed until imaging, while a bleed identified at 25 h cannot be definitively classified as occurring before or after the 24-h threshold. Accordingly, this study was not intended to determine the precise pathophysiologic onset of bleeding but instead used the measurable timing of brain CT scans in real-world practice as a pragmatic proxy for asICH detection. The 24-h and 48-h scenarios therefore represent retrospective categorizations rather than a prospective comparison of two fixed time-point strategies, which would be ethically challenging to conduct. By applying a multistate illness-death model [13] - a method rarely used in published research due to its complexity - we were able to more accurately capture outcome transitions and simulate cumulative incidence functions (Fig. 3), providing insights that conventional binary analyses, which ignore the time dimension, cannot reveal.
In Thailand, patients treated with IV alteplase generally remain hospitalized for at least 72 h, during which neurological monitoring is continuous. Introducing a 48-h follow-up brain CT scan would not increase clinical risk, as any acute neurological worsening during this period would already trigger immediate imaging according to standard protocols. Thus, adopting a 48-h schedule may strengthen detection of silent hemorrhagic events without compromising patient safety.
This study has several limitations. First, as a retrospective study, missing data were unavoidable. However, the timing of CT scans - our primary variable - was objective, complete, and reliable. In contrast, the timing of clinical deterioration, which reflects sICH, was recorded by nurses and may be less precise. This was not a major concern, as our primary outcome was asICH, which does not require precise timing of clinical deterioration. Second, follow-up brain CT scans were not performed at uniform time points; scan timing often depended on clinical judgment and logistical constraints, which may have influenced detection rates. Third, distinguishing asICH from sICH requires complete NIHSS data, which were missing in some cases. To minimize misclassification, we incorporated contextual clinical information such as progression of motor power deficit and other newly documented neurological signs by physicians, or subsequent neurosurgical intervention. Fourth, although the numbers of asICH and sICH exceeded the predefined requirements, the no ICH group was slightly smaller than expected. Nevertheless, the observed ratios of no ICH to sICH (14:1) and no ICH to asICH (9:1) remained close to the target design (16:1 and 10:1, respectively), suggesting that the impact on study power was minimal. Finally, this was a single-center study, which may limit the generalizability of our findings to other settings and populations, particularly outside of Asian cohorts where delayed bleeding is disproportionately more frequent. A multi-center study applying the multistate illness-death model would be essential to validate and extrapolate these findings. Future research may also explore cost-effectiveness, long-term outcomes, and external validation across diverse stroke populations to strengthen generalizability.
Conclusions
Extending follow-up brain CT scan from 24 to 48 h after IV alteplase markedly improves the detection of asICH without additional radiation risk or resource burden. By applying a multistate illness-death model, we demonstrated dynamic transitions that conventional analyses often overlook, emphasizing the clinical relevance of delayed bleeding, particularly in Asian populations. These findings support reconsideration of current imaging protocols, although multicenter studies are needed to validate whether a 48-h strategy should replace the conventional 24-h scan.
Acknowledgments
This study was supported by the Lampang Medical Education Center. We gratefully acknowledge the Emergency Medicine residents, staff, and nurses of the Department of Emergency Medicine, Lampang Hospital, along with the Stroke Unit team, for their invaluable contributions.
Financial Disclosure
This study was supported by the Lampang Medical Education Center.
Conflict of Interest
The authors reported no contents in the article as conflict of interest.
Informed Consent
Informed consent was waived due to the observational nature of the study.
Author Contributions
Conceptualization: Pranisa Botkosa, Thanin Lokeskrawee, Donsuk Pongnikorn, Suppachai Lawanaskol, Jayanton Patumanond. Data curation: Pranisa Botkosa. Formal analysis: Natthaphon Pruksathorn, Pranisa Botkosa, Thanin Lokeskrawee, Donsuk Pongnikorn, Suppachai Lawanaskol, Jayanton Patumanond, Jarupa Yaowalaorng, Suwapim Chanlaor, Wanwisa Bumrungpagdee, Chawalit Lakdee. Methodology: Thanin Lokeskrawee, Donsuk Pongnikorn, Natthaphon Pruksathorn, Suppachai Lawanaskol, Jayanton Patumanond. Supervision: Thanin Lokeskrawee, Donsuk Pongnikorn, Suppachai Lawanaskol, Jayanton Patumanond. Writing - original draft: Pranisa Botkosa, Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AIS: acute ischemic stroke; AHA: American Heart Association; asICH: asymptomatic intracranial hemorrhage; BMI: body mass index; CI: confidence interval; CT: computed tomography; ED: Emergency Department; HI: hemorrhagic infarction; ICH: intracranial hemorrhage; IQR: interquartile range; IV: intravenous; MT: mechanical thrombectomy; NIHSS: National Institutes of Health Stroke Scale; OPD: Outpatient Department; rt-PA: recombinant tissue plasminogen activator; sICH: symptomatic intracranial hemorrhage; SD: standard deviation; TCTR: Thai Clinical Trials Registry; TOAST: Trial of ORG 10172 in Acute Stroke Treatment
| References | ▴Top |
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587.
doi pubmed - Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438-441.
doi pubmed - Suwanwela NC, Phanthumchinda K, Likitjaroen Y. Thrombolytic therapy in acute ischemic stroke in Asia: The first prospective evaluation. Clin Neurol Neurosurg. 2006;108(6):549-552.
doi pubmed - Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
doi pubmed - Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, Erila T, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke. 2008;39(12):3316-3322.
doi pubmed - Dharmasaroja PA, Muengtaweepongsa S, Pattaraarchachai J, Dharmasaroja P. Intracerebral hemorrhage following intravenous thrombolysis in Thai patients with acute ischemic stroke. J Clin Neurosci. 2012;19(6):799-803.
doi pubmed - Chen PM, Lehmann B, Meyer BC, Rapp K, Hemmen T, Modir R, Agrawal K, et al. Timing of symptomatic intracerebral hemorrhage after rt-PA treatment in ischemic stroke. Neurol Clin Pract. 2019;9(4):304-308.
doi pubmed - Lokeskrawee T, Muengtaweepongsa S, Patumanond J, Tiamkao S, Thamangraksat T, Phankhian P, Pleumpanupatand P, et al. Prognostic parameters for symptomatic intracranial hemorrhage after intravenous thrombolysis in acute ischemic stroke in an Asian Population. Curr Neurovasc Res. 2017;14(2):169-176.
doi pubmed - Lokeskrawee T, Muengtaweepongsa S, Patumanond J, Tiamkao S, Thamangraksat T, Phankhian P, Pleumpanupat P, et al. Prediction of symptomatic intracranial hemorrhage after intravenous thrombolysis in acute ischemic stroke: the symptomatic intracranial hemorrhage score. J Stroke Cerebrovasc Dis. 2017;26(11):2622-2629.
doi pubmed - Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418.
doi pubmed - Pengtong W, Aimyong N, Nilanont Y. Intracranial hemorrhage after recombinant tissue plasminogen Activator: The competing risks survival analysis. Interdiscip Neurosurg. 2023;32:101734.
- Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41.
doi pubmed - Posch F, Leitner L, Bergovec M, Bezan A, Stotz M, Gerger A, Pichler M, et al. Can multistate modeling of local recurrence, distant metastasis, and death improve the prediction of outcome in patients with soft tissue sarcomas? Clin Orthop Relat Res. 2017;475(5):1427-1435.
doi pubmed - Neuberger U, Mohlenbruch MA, Herweh C, Ulfert C, Bendszus M, Pfaff J. Classification of bleeding events: comparison of ECASS III (European Cooperative Acute Stroke Study) and the New Heidelberg Bleeding Classification. Stroke. 2017;48(7):1983-1985.
doi pubmed - Hong JM, Kim DS, Kim M. Hemorrhagic transformation after ischemic stroke: mechanisms and management. Front Neurol. 2021;12:703258.
doi pubmed - Honig A, Percy J, Sepehry AA, Gomez AG, Field TS, Benavente OR. Hemorrhagic transformation in acute ischemic stroke: a quantitative systematic review. J Clin Med. 2022;11(5):1260.
doi pubmed - Choi HY, Cho Y, Kim W, Minn YK, Kang GH, Jang YS, Lee Y, et al. Analysis of mortality in intracerebral hemorrhage patients with hyperacute ischemic stroke treated using thrombolytic therapy: a nationwide population-based cohort study in South Korea. J Pers Med. 2022;12(8):e0272257.
doi pubmed - Teekaput C, Thiankhaw K, Tanprawate S, Teekaput K, Chai-Adisaksopha C. Outcomes of asymptomatic recombinant tissue plasminogen activator associated intracranial hemorrhage. PLoS One. 2022;17(8):e0272257.
doi pubmed - Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(12):e343-e361.
doi pubmed - Maier B, Desilles JP, Mazighi M. Intracranial hemorrhage after reperfusion therapies in acute ischemic stroke patients. Front Neurol. 2020;11:599908.
doi pubmed - Lei YS, Li H, Lei JY, Li SX, Li DF. Effect of intravenous thrombolysis in acute ischemic stroke patients with cerebral microbleeds and analysis of risk factors for hemorrhagic transformation. Eur Rev Med Pharmacol Sci. 2022;26(3):779-786.
doi pubmed - Thomas SE, Plumber N, Venkatapathappa P, Gorantla V. A review of risk factors and predictors for hemorrhagic transformation in patients with acute ischemic stroke. Int J Vasc Med. 2021;2021:4244267.
doi pubmed - Mehta RH, Cox M, Smith EE, Xian Y, Bhatt DL, Fonarow GC, Peterson ED, et al. Race/Ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke. 2014;45(8):2263-2269.
doi pubmed - Kim HK, Tantry US, Smith SC, Jr., Jeong MH, Park SJ, Kim MH, Lim DS, et al. The East Asian Paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. 2021;121(4):422-432.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.