| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 12, December 2025, pages 698-707
Ruxolitinib Plus Extracorporeal Photopheresis for Steroid-Refractory Acute and Chronic Graft-Versus-Host Disease
Khalid Halahleha, g , Iyad Sultanb, Ahmad Abu-Khaderc, Rozan Al-Fara, Lina Halahlehd, Zaid Abdel Rahmana, Isra Muradie, Eman Khattabf, Husam Abu Jazara
aDepartment of Internal Medicine, Adult Bone Marrow Transplantation and Cellular Therapy Program, King Hussein Cancer Center, Amman, Jordan
bDepartment of Pediatrics, Medical Oncology Hematology, King Hussein Cancer Center, Amman, Jordan
cDepartment of Cell Therapy and Applied Genomics, King Hussein Cancer Center, Amman, Jordan
dAl-Quds University, College of Health Sciences, Jerusalem, Palestine
eAl-Ahliyya Amman University (AAU), Al-Salt, Jordan
fDepartment of Pediatrics, Pediatric Bone Marrow Transplantation and Cellular Therapy Program, King Hussein Cancer Center, Amman, Jordan
gCorresponding Author: Khalid Halahleh, Department of Internal Medicine, Adult Bone Marrow Transplantation and Cellular Therapy Program, King Hussein Cancer Center, Amman 11941, Jordan
Manuscript submitted September 11, 2025, accepted November 20, 2025, published online December 24, 2025
Short title: Ruxo + ECP vs. Ruxo Alone in SR-GvHD
doi: https://doi.org/10.14740/jocmr6385
| Abstract | ▴Top |
Background: Graft-versus-host disease (GvHD) is a serious complication of allogeneic hematopoietic cell transplantation, and the major cause of post-transplant mortality and morbidity. If steroid treatment as first-line therapy fails, treatment options are limited. Ruxolitinib (Ruxo) as well as extracorporeal photopheresis (ECP) showed high efficacy in the treatment of steroid-refractory (SR) acute and chronic GvHD.
Methods: We interrogated data from 68 adult and pediatric patients with SR acute and chronic GvHD, between 2017 and 2024, who received either Ruxo plus ECP (Ruxo + ECP, n = 31) or Ruxo alone (Ruxo, n = 37). Endpoints were to compare the overall response rates (ORRs) including complete response (CR) and partial response (PR) of acute and chronic GvHD at last encounter, and the percentage of patients with history of acute GvHD, who progressed to chronic GvHD at 1 year, 1-year non-relapse mortality (NRM), graft-versus-host disease relapse-free survival (GRFS) and survival outcomes at 3 years.
Results: Patient, disease, and transplant characteristics were well balanced, except for more severe acute GvHD in Ruxo + ECP arm (66.6% vs. 18.5%, P = 0.007) and longer Ruxo treatment in Ruxo alone arm (11 vs. 7 months, P = 0.05). The ORRs were 58% for Ruxo + ECP arm compared to 49% in Ruxo alone arm (P = 0.002) at last encounter and the duration of response was 17.6 versus 9 months (P = 0.3171), respectively. In both arms, 87% and 93% of patients could taper steroids rapidly by 50% and 16%. At 1 year, cumulative incidence of chronic GvHD was higher after Ruxo versus Ruxo + ECP, being 55% (95% CI: 42-69%) vs. 26% (95% CI: 22-64%) (P = 0.018). No statistically significant difference in 1-year NRM, relapse, and GRFS and survival at 3 years was observed.
Conclusion: Our data suggest improved long-term control of acute and chronic GvHD by combining Ruxo plus ECP compared with Ruxo alone.
Keywords: Steroid-refractory graft-versus-host disease; GvHD; Ruxolitinib; Extracorporeal photopheresis; Allogeneic hematopoietic cell transplantation
| Introduction | ▴Top |
Allogeneic hematopoietic cell transplants (allo-HCTs) are increasing with 20,485 transplant procedures reported by the European Society for Blood and Marrow Transplantation (EBMT) in 2023 [1]. Acute graft-versus-host disease (aGvHD) is a serious frequent complication of allo-HCT, and the major cause of post-transplant non-relapse mortality and morbidity. Though the survival rates after allo-HCT have significantly improved over the past decades, the rate of developing acute and chronic GvHD remains significantly high, ranging between 40-50% and 20-25%, respectively [2]. GvHD is an alloreactive immune response caused by donor T lymphocytes which are activated by host antigen-presenting cells, leading to an inflammatory response [3]. Upper and lower gastrointestinal (GI) tract, skin, and liver are the most predominantly affected organs. Chronic graft-versus-host disease (cGvHD) characterized by a variety of clinical manifestations, most often involves the skin and mouth, but almost any other organ system can be involved [4]. Despite the advancement in GvHD prophylaxis over the last decade with the introduction and approval of new modality including post-transplant cyclophosphamide (PTCy) and novel agents like ruxolitinib (Ruxo) and others [4], GvHD can happen in a high percentage of patients even in matched related transplants, depending on recipient’s and donor characteristics, such as recipient and donor age, graft source or underlying disease and donor type, conditioning intensity and the type of GvHD prophylaxis, and others [2, 5]. There are different treatment strategies available for the treatment of acute and chronic GvHD [6-8]. Corticosteroids are the mainstay of treatment and response to steroids is a key predictor of clinical outcome [6]. Standard therapy for aGvHD is 2 mg methylprednisolone per kilogram bodyweight (or equivalent dose of prednisolone) and 0.5 - 1 mg/kg/day for cGvHD. Thirty to forty percent will respond to steroids with long durable remission [8-11]. The outcome of steroid-refractory GvHD (SR-GvHD) remains poor. Due to the limited efficacy of second- and third-line therapies in these cases, new approaches are needed [10].
Ruxo is a selective, small molecule Janus kinase (JAK) 1/2 inhibitor. It causes a blockade of the JAK-STAT pathway which is, among a lot of other effects, known to play a role in T effector cell responses [11, 13]. Zeiser et al reported a clinical multicenter survey on 54 patients who received Ruxo in SR-aGvHD and 41 patients who received Ruxo in SR-cGvHD, showing an encouraging overall response rate (ORR) of 82% and 85%, respectively, with low relapse rates of 7% and 6% [13]. Ruxo was an approved treatment for patients with SR-aGvHD, based on the reported long-term results of an open-label, multicenter phase III study (NCT02913261, REACH2) and for patients with cGvHD based on the outcomes of a phase III clinical study (NCT02913261, REACH-III), which compared Ruxo therapy versus best available treatment in SR-aGvHD and cGvHD after allotransplant [14, 16]. Also, Locatelli et al reported on the benefits of Ruxo in pediatric patients with treatment-naive and SR-aGvHD (REACH4, #NCT03491215) with an ORR of 84.4% at day 28, with a durable ORR at day 56 of 66.7%, and high response rates were observed across age groups and in both treatment-naive and SR subgroups with no new safety signals [16]. Ruxo therapy is an innovative treatment of GvHD, but with severe adverse effects, particularly in combination with other immunosuppressive therapies, including prolonged pancytopenia attended by severe infections and bleeding complications [17].
Extracorporeal photopheresis (ECP) is another therapeutic modality for acute and chronic GvHD, using ultraviolet A (UV-A) light in combination with 8-methoxypsoralen to induce apoptosis of leukapheresis-gained mononuclear cells. Safety and efficacy of ECP was confirmed in several retrospective and prospective clinical trials as second- or third-line treatment in acute and chronic GvHD with an objective response of 60-87% [18-25]. We reported 42 patients with aGvHD (n = 34; 25 grade III-IV) and moderate to severe cGvHD (n = 23) with an objective response of 38% and 58%, respectively [24].
Here, we report our experience on efficacy, safety, and tolerability of combining both therapeutic strategies - treatment with Ruxo in combination with ECP (Ruxo + ECP) and compare with patients receiving Ruxo alone in patients with SR acute and chronic GvHD.
| Materials and Methods | ▴Top |
Study design, patients, and definitions
We used the Bone Marrow Transplant Program Registry of King Hussein Cancer Center (KHCC) to identify 68 patients with SR acute and chronic GvHD, between 2017 and 2024, who received either Ruxo + ECP (n = 31) or Ruxo alone (n = 37). Patients were allocated to either arm based on the availability of ECP in early years, presence of severe infection and/or pancytopenia, and the approval of Ruxo in later years in our center. ECP was provided using a closed system, Therakos Cellex machine. The process started by collection of fraction of blood by apheresis, then separated by centrifugation by selecting mononuclear cells, through an intravenous central catheter; red blood cells and plasma were returned to the patient. Once the T cells were separated, a photosensitizing drug, UVADEX® (methoxsalen), was a photosensitizing agent added to the buffy coat fraction and cells were photoactivated by ultraviolet (UV) light exposure. The photoactivated buffy coat fraction was then reinfused to the patient to induce an immune-modulating effect and apoptosis.
All adult patients received Ruxo at a dose of 10 mg twice daily in addition to calcineurin inhibitor and steroid therapy. In pediatric patients (≤ 18 years), Ruxo dosing was based on age and targeted the exposure in adults receiving 10 mg twice daily; group 1 (aged ≥ 12 to < 18 years) received 10 mg twice daily and preliminary starting doses for groups 2 (aged ≥ 6 to < 12 years) and 3 (aged ≥ 2 to < 6 years) were 5 mg twice daily and 4 mg/m2 twice daily, respectively [16]. Dose modifications of Ruxo were done according to Ruxo guidelines in case of cytopenia or severe infections and steroids were tapered rapidly every 3 - 5 days schedule in aGvHD and every 5 - 7 days in cGvHD. ECP was initiated either twice or three times weekly for patients with aGvHD for the first 2 weeks then twice every other week, then individual reduction of ECP frequency. Patients with cGvHD received two sessions every 2 weeks for the first 12 weeks (induction), then two sessions every 4 weeks for the rest of ECP therapy. The median number of ECP cycles was 15 (range: 7 - 115).
In the first step, we reported responses and survival outcomes for patients who received Ruxo + ECP and then we compared patients, disease, transplant, GvHD characteristics and treatment outcomes with those who received Ruxo alone.
Endpoints
The primary objective of this study was to compare the ORR (complete response (CR) and partial response (PR)) of SR acute and chronic GvHD at last encounter and then compare the two arms. The secondary endpoints were the percentage of patients with history of aGvHD, who progressed to cGvHD later in the course of the disease, 1-year non-relapse mortality (NRM), graft-versus-host disease relapse-free survival (GRFS), and survival outcomes. Kaplan-Meier estimates of survival and cumulative incidences of NRM, acute and chronic GvHD were tested by log-rank and grey tests, respectively. Survival outcomes were given at 3 years after allotransplant. aGvHD was staged and graded according to MAGIC criteria and cGvHD based on NIH criteria respectively [25, 28]. SR-GvHD was defined as progression after 3 days or no clinical improvement of aGvHD after 7 days of initial treatment with steroids of greater than 2 mg/kg per day or an inability to taper the steroid dose to < 0.5 mg/kg [25]. SR-cGvHD is defined by failure to respond to high-dose steroids or worsening disease while on them. Specifically, it means the disease progresses despite treatment with 1 mg/kg/day of prednisone for at least 1 week, persists despite 0.5 mg/kg/day for at least 1 month, or flares up when the dose is tapered below 0.25 mg/kg/day [26].
Definitions
ORR was defined as proportion of patients achieving CR and PR without additional systemic immunosuppressive therapy. CR was defined as the absence of aGvHD symptoms and/or signs in all organs involved without increased immunosuppressive therapy and PR was defined as a decrease in the initially affected organs of GvHD of at least 1 grade without a new manifestation or worsening of at least 1 grade in any other organ. No-response (NR) was defined as no change in GvHD severity in the affected organs of GvHD, and progression was defined as an increase of GvHD grade in at least one organ with or without improvement in any other organs. GRFS was defined as no relapse, no acute GvHD or moderate to severe cGvHD, requiring systemic therapy [24].
The Institutional Review Board (IRB) of KHCC approved the study and waived the informed consent (No. 25 KHCC 032). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistical analysis
Descriptive statistics were used for patient demographics. Quantitative baseline variables were described as median and qualitative described as numbers and percentages. Incidences of acute and chronic GvHD were estimated considering relapse and NRM as competing risks [27]. NRM and cumulative incidence of relapse (CIR) and GvHD were analyzed using a competing risk model [28]. Survival was estimated using Kaplan-Meier method calculated from the date of the start of Ruxo treatment to death from any cause, censored at the last follow-up. NRM was defined as death without evidence of disease relapse. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). P-values are two-sided and considered significant when < 0.05.
| Results | ▴Top |
Patient, disease, and transplant characteristics are detailed in Table 1. Sixty-eight patients were included in the analysis. Thirty-one patients received Ruxo + ECP and 37 Ruxo alone.
 Click to view | Table 1. Patient, Disease, and Transplant Characteristics |
Patients’ demographics in the Ruxo + ECP arm: 22 patients (71%) were adults and 19 (61%) were males with a median age of 34 (range: 18 - 62) years in adults, and 13 (2 - 17) years in pediatric group. Eighteen patients (70%) had acute leukemia. Eighteen patients (58%) received intensive pretransplant conditioning and 31 (100%) received a blood cell graft. Twenty-two (71%) of donors were HLA-matched relatives, and nine (29%) were mismatched relatives. Four patients (6%) received post-transplant cyclophosphamide (PTCy).
Twenty-seven patients (87%) developed aGvHD which included 25 (96%) with ≥ grade 2, at a median of 58 days (interquartile range (IQR): 32 - 127) after allotransplant. Seventeen patients (63%) had gut aGvHD, 14 (52%) with grade III/IV. Twenty-four patients (77%) developed cGvHD, which was moderate to severe in 23 (74%), at a median of 337 days (IQR: 197 - 491) after allotransplant. Twenty-four patients (89%) had SR-GvHD, and seven (26%) could not taper steroid < 0.5 mg/kg. Twenty-nine patients (93%) received calcineurin inhibitor, and on steroids therapy. The median duration of Ruxo was 7.3 months (range: 1 - 28) and patients received a median of 33 ECP cycles (range: 7 - 115).
Comparison between the two cohorts
The median ages in the Ruxo + ECP arm in adult and pediatric arms were 34 (range: 18 - 62) and 28 (range: 18 - 59) years and 13 (2 - 17) and 11.5 (6 - 17) years, respectively in Ruxo arm. Patient, disease, and transplant characteristics were well balanced (Table 1). The type of SR-aGvHD was well balanced between both arms except that severe aGVHD grade III-IV was observed higher in Ruxo + ECP arm (66.6% vs. 18.5%; P = 0.007) and the duration of Ruxo therapy was numerically longer in Ruxo alone arm (11 vs. 7 months, P = 0.05). GvHD characteristics of both arms are shown in Table 2.
 Click to view | Table 2. GvHD Characteristics in Both Arms |
Responses
GvHD and patient outcomes are detailed in Table 3. At last encounter, ORRs were 58% for Ruxo + ECP arm compared to 49% in Ruxo alone arm (P = 0.002) and the median duration of response was not reached in either arm with no statistically significant difference between the two arms (17.6 vs. 9 months; P = 0.3171), respectively. After patients responded, 87% in Ruxo + ECP and 93% in Ruxo alone arm could taper steroids rapidly by 50%. When we analyzed patients according to age (adult versus pediatric), 80% and 75% in Ruxo + ECP arm, and 100% and 80% in the Ruxo alone arm responded (P = NS). Analyzing patients with gut aGvHD (n = 24; 20 grade II/IV), 17 patients received Ruxo + ECP, and six received Ruxo alone. Ten out of 17 (59%) responded in Ruxo + ECP and no responses (n = 6) in Ruxo alone arm.
 Click to view | Table 3. Outcomes |
At 6 and 12 months, 20% and 26% in the Ruxo + ECP arm and 40% and 55% in the Ruxo alone arm of evaluable patients experienced cGvHD, resulting in a 1-year cumulative incidence of cGvHD of 26% (95% CI: 22-64%) after Ruxo + ECP and 55% (95% CI: 41.94-69%) after Ruxo alone (P = 0.33). At 1 year, 20 out of 27 (74%) evaluable patients after Ruxo + ECP experienced cGvHD compared to 16 out of 27 (59%) in the Ruxo alone arm with no statistically significant P value (0.387). Also, at 1 year, five patients (16%) in Ruxo + ECP arm continued to receive immunosuppression compared to seven (19%) in Ruxo alone arm.
There was no statistically significant difference in NRM (19% (95% CI: 5-55%) vs. zero (P = 0.31) with unadjusted hazard ratio of 1.09 (95% CI: 0.28 - 4.26; P = 0.905), probably related to small sample size and CIR (6% vs. 2%) between Ruxo + ECP (95% CI: 8 - 33) and Ruxo alone arms (95% CI: 2 - 13, P > 0.5) at 1 year, respectively. No statistically significant difference in GRFS at 3 years was observed in both arms calculated from the Ruxo start date (94.7% (95% CI: 95-100%) versus 100% (95% CI: 100-100%); P = 0.23).
Post-therapy complications in Ruxo + ECP and Ruxo alone arms included cytomegalovirus (CMV) reactivation (14 (45%) vs. 15 (45%)), bacterial infections (13 (42%) vs. 7 (19%)), and fungal infections (3 (10%) vs. 1 (3%)) and four (13%) patients developed post-ECP thrombocytopenia. Common causes of transplant-related mortality in Ruxo + ECP arm were GvHD (n = 6) and infection with meningoencephalitis (n = 1).
Survival outcomes
After a median follow-up of 27 months (IQR: 17.4 - 56.5) for the whole patients population, 34.3 months (IQR: 2.5 - 159.2) for Ruxo + ECP and 24.3 months (IQR: 0.8 - 111.7) for Ruxo arms (P = 0.078), 94% and 97% respectively, were in complete remission of the primary disease; 78% and 95% are alive, respectively. Survival for the whole cohort (n = 68) at 3 years was 78.3% (95% CI: 66-93%). Three-year survival was 70% (95% CI: 53-92%) for Ruxo + ECP and 80% (95% CI: 65-87%) for Ruxo alone arms, respectively (P = 0.36; Fig. 1 and Table 3). There was no statistically significant difference between adult and pediatric age groups with an interaction test ≥ 0.10. Also, there was no difference in survival when adjusted to age and sex (all P > 0.1).
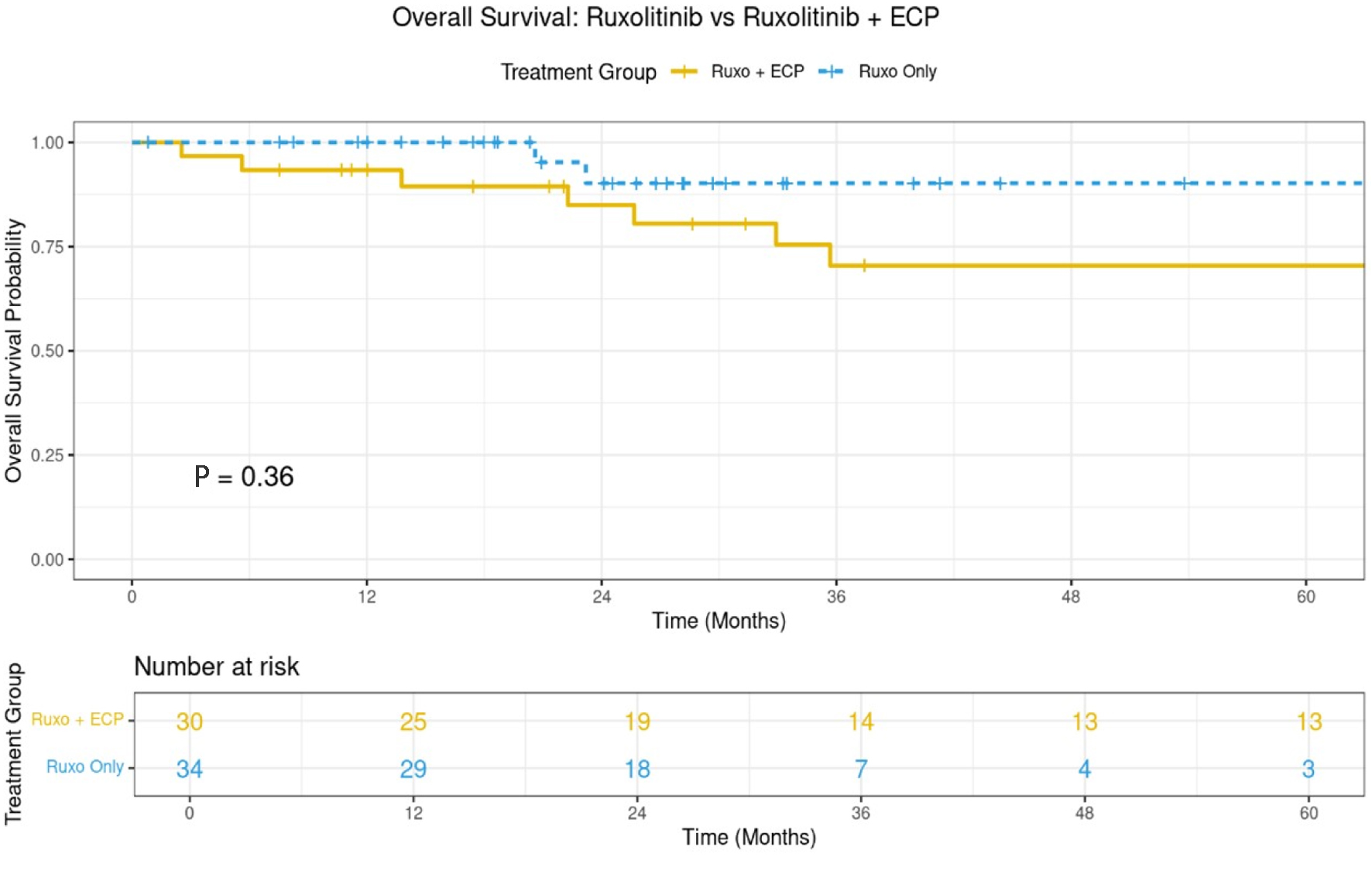 Click for large image | Figure 1. The 3-year overall survival for both arms (n = 68). |
| Discussion | ▴Top |
In this single-center study from KHCC, we included severely affected patients with SR acute and chronic GvHD with more than 70% of reported cases having high grade GvHD and two-thirds of patients having primary progressive GvHD. Combination of Ruxo and ECP resulted in a higher ORR (58%), CR (39%), and PR (19%) and an encouraging 3-year survival of 70% in those patients who could reach CR or PR. In our analysis, ORR among all patients included is 53% which is below the published data [13, 16, 17, 31, 32]. Also, responses in the Ruxo alone cohort are lower than reported in clinical trials and real-world data. Our results contradict reported real-world data, expanded access programs [31- 34]. The lower ORR and CR rates could be explained by including severely affected patients with high grade GvHD.
While these studies confirmed efficacy and safety of Ruxo in SR-aGvHD, long-term follow-up data and subsequent development of cGvHD are limited. Lastovytska et al reported an incidence of cGvHD of 24% and Leung et al reported an incidence of cGvHD of 20% in patients who achieved CR of aGvHD after Ruxo treatment [30, 33]. Patients with aGvHD can respond initially but progress later during the disease [14]. Long-term control of aGvHD is a vital objective and there is unmet need for better long-term control of SR-aGvHD. As a result, combination therapy has become of clinical interest and few studies were published [29-33]. Also, other studies have reported that ECP alone represents a high-potential treatment for patients with SR-GvHD with ORR of 60-75% [18-26].
In this study, we observed a higher ORR and PR in Ruxo + ECP arm compared with Ruxo alone, despite including more patients with higher grade SR-GvHD, but similar CR rates in both arms. Higher ORR was observed in gut aGvHD treated with Ruxo + ECP (P = 0.001) and fewer patients with no response. Though, there was no statistically significant difference between Ruxo + ECP vs. Ruxo alone in the percentage of patients with history of aGvHD, who progressed to cGvHD after achieving CR (74% vs. 59%, P = 0.387), the incidence of cGvHD was significantly higher after Ruxo alone compared with Ruxo + ECP arm (55% vs. 26%, P = 0.018). While 1-year NRM was significantly lower in Ruxo alone arm (0 vs. 19%, P = 0.006), which is probably related to small sample size, there was no statistically significant differences in 3-year survival (P = 0.36) or GRFS (P = 0.23) between the two arms.
Our study has several limitations. First, this is a retrospective study with small number of patients. Second, GvHD characteristics are not well balanced between the GvHD severity: the proportion of patients with higher grade (III-IV) aGvHD and moderate to severe cGvHD was higher in the Ruxo + ECP cohort compared to Ruxo alone cohort. All patients intended to start both therapies simultaneously, but there was a time gap between the start of the two treatments in some patients in the combination of Ruxo plus ECP due to logistic reasons, which might affect the fair comparison between the two study arms.
Conclusion
Our data suggest better long-term control of acute and chronic GvHD by combining Ruxo plus ECP compared with Ruxo alone. Long follow-up time, high ORR particularly in severe gut aGvHD, and low NRM strengthen the study results. Our data might have better insight into other retrospective studies so far published but need to be confirmed in a multicenter multinational and a prospective randomized trial.
Acknowledgments
The authors express gratitude to all patients and healthcare providers.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The study was approved, and the consent was granted exemption by the IRB of KHCC (No. 25 KHCC 032).
Author Contributions
KH has the concept and design of the study and wrote the first draft and final typescript, RA, LH, ZA, HA, AA, IM, and EK extracted the data and contributed to data clearance. IS completed analysis and interpretation of the data. All authors edited the initial draft and provided feedback. The authors approved the final typescript, took responsibility for the content, and agreed to submit it for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
aGvHD: acute graft-versus-host disease; Allo-HCT: allogeneic hematopoietic cell transplant; cGvHD: chronic graft-versus-host disease; EBMT: European Society for Blood and Marrow Transplantation; ECP: extracorporeal photopheresis; GRFS: graft-versus-host disease relapse-free survival; GvHD: graft-versus-host disease; KHCC: King Hussein Cancer Center; NRM: non-relapse mortality; ORR: overall response rate; PTCy: post-transplant cyclophosphamide; Ruxo: ruxolitinib; SR: steroid-refractory
| References | ▴Top |
- Passweg JR, Baldomero H, Atlija M, Kleovoulou I, Witaszek A, Alexander T, Angelucci E, et al. The 2023 EBMT report on hematopoietic cell transplantation and cellular therapies. Increased use of allogeneic HCT for myeloid malignancies and of CAR-T at the expense of autologous HCT. Bone Marrow Transplant. 2025;60(4):519-528.
doi pubmed - Jaglowski SM, Devine SM. Graft-versus-host disease: why have we not made more progress? Curr Opin Hematol. 2014;21(2):141-147.
doi pubmed - Zhang L, Yu J, Wei W. Advance in targeted immunotherapy for graft-versus-host disease. Front Immunol. 2018;9:1087.
doi pubmed - Asensi Canto P, Gomez-Segui I, Montoro J, Villalba Montaner M, Chorao P, Solves Alcaina P, Santiago Balsera M, et al. Incidence, risk factors and therapy response of acute graft-versus-host disease after myeloablative hematopoietic stem cell transplantation with posttransplant cyclophosphamide. Bone Marrow Transplant. 2024;59(11):1577-1584.
doi pubmed - Bhatt VR, Wang T, Chen K, Kitko CL, MacMillan ML, Pidala JA, Al Malki MM, et al. Chronic Graft-versus-Host Disease, Nonrelapse mortality, and disease relapse in older versus younger adults undergoing matched allogeneic peripheral blood hematopoietic cell transplantation: a center for international blood and marrow transplant research analysis. Transplant Cell Ther. 2022;28(1):34-42.
doi pubmed - Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, Giebel S, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11(2):e147-e159.
doi pubmed - Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150-1163.
doi pubmed - Cesen Mazic M, Girandon L, Knezevic M, Avcin SL, Jazbec J. Treatment of severe steroid-refractory acute-Graft-vs.-Host disease with mesenchymal stem cells-single center experience. Front Bioeng Biotechnol. 2018;6:93.
doi pubmed - Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, Khouri IF, et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol. 2011;2011:601953.
doi pubmed - Schechter T, Macartney C, Finkelstein Y, Gassas A, Husain M, Doyle J, Dupuis LL. Dismal response to high-dose methylprednisolone after failure to respond to standard dose in patients with acute GVHD. Bone Marrow Transplant. 2010;45(12):1749-1753.
doi pubmed - Bousoik E, Montazeri Aliabadi H. "Do We Know Jack" about JAK? A closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
doi pubmed - Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, Verbeek M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832-3842.
doi pubmed - Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062-2068.
doi pubmed - Mohty M, Socie G, Szer J, Niederwieser D, Butler J, Wagner-Drouet E, Or R, et al. Ruxolitinib versus best available therapy in patients with steroid-refractory acute Graft-versus-Host disease: final analysis from the randomized phase III REACH2 trial. J Clin Oncol. 2025;43(34):3639-3645.
doi pubmed - Zeiser R, Russo D, Ram R, Hashmi SK, Chakraverty R, Middeke JM, Musso M, et al. Ruxolitinib in patients with corticosteroid-refractory or corticosteroid-dependent chronic Graft-versus-Host disease: 3-year final analysis of the phase III REACH3 study. J Clin Oncol. 2025;43(23):2566-2571.
doi pubmed - Locatelli F, Kang HJ, Bruno B, Gandemer V, Rialland F, Faraci M, Takahashi Y, et al. Ruxolitinib for pediatric patients with treatment-naive and steroid-refractory acute graft-versus-host disease: the REACH4 study. Blood. 2024;144(20):2095-2106.
doi pubmed - Saeed I, McLornan D, Harrison CN. Managing side effects of JAK inhibitors for myelofibrosis in clinical practice. Expert Rev Hematol. 2017;10(7):617-625.
doi pubmed - Lozano M, Charry P, de Pablo-Miro M, Salas MQ, Martinez C, Suarez-Lledo M, Fernandez-Aviles F, et al. Role of extracorporeal photopheresis in the management of acute and chronic graft versus disease: current status. Bone Marrow Transplant. 2024;59(9):1209-1214.
doi pubmed - Gavriilaki E, Papchianou E, Karavalakis G, Batsis I, Panteliadou A, Lazaridou A, Mallouri D, et al. Safety and efficacy of extracorporeal photopheresis for acute and chronic graft-versus-host disease. Pharmaceuticals (Basel). 2024;17(10):1279.
doi pubmed - Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, Bacigalupo A, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112(7):2667-2674.
doi pubmed - Asensi Canto P, Sanz Caballer J, Solves Alcaina P, de la Rubia Comos J, Gomez Segui I. Extracorporeal photopheresis in Graft-versus-Host disease. Transplant Cell Ther. 2023;29(9):556-566.
doi pubmed - Solh MM, Farnham C, Solomon SR, Bashey A, Morris LE, Holland HK, Zhang X. Extracorporeal photopheresis (ECP) improves overall survival in the treatment of steroid refractory acute graft-versus-host disease (SR aGvHD). Bone Marrow Transplant. 2023;58(2):168-174.
doi pubmed - Greinix HT, Ayuk F, Zeiser R. Extracorporeal photopheresis in acute and chronic steroid-refractory graft-versus-host disease: an evolving treatment landscape. Leukemia. 2022;36(11):2558-2566.
doi pubmed - Rihani R, Halahleh K, Abu Shanap M, Khattab E, Hashem H, Sultan I. Extracorporeal photopheresis for acute and chronic GvHD in pediatric HCTs: insights from King Hussein Cancer Center. Transplantation and Cellular Therapy. 2024;30(2):S280.
- Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, et al. International, multicenter standardization of acute Graft-versus-Host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10.
doi pubmed - Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, et al. National institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-Host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e381.
doi pubmed - Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381-387.
doi pubmed - Iacobelli S, Committee ES. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48(Suppl 1):S1-37.
doi pubmed - Modemann F, Ayuk F, Wolschke C, Christopeit M, Janson D, von Pein UM, Kroger N. Ruxolitinib plus extracorporeal photopheresis (ECP) for steroid refractory acute graft-versus-host disease of lower GI-tract after allogeneic stem cell transplantation leads to increased regulatory T cell level. Bone Marrow Transplant. 2020;55(12):2286-2293.
doi pubmed - Lastovytska I, Heidenreich S, Klyuchnikov E, Niederwieser C, Gagelmann N, Richter J, Massoud R, et al. Lower incidence of chronic graft-versus-host disease after ruxolitinib plus extracorporeal photopheresis versus ruxolitinib alone in steroid-refractory acute graft-versus-host disease following allogeneic stem cell transplantation. Haematologica. 2025;110(7):1536-1544.
doi pubmed - Leung GMK, Sim JPY, Hwang YY, Chan TSY, Lie AKW, Tse E, Kwong YL. Ruxolitinib in the management of steroid-resistant/-dependent acute and chronic graft-versus-host disease: results of routine practice in an academic centre. Ann Hematol. 2022;101(1):155-163.
doi pubmed - Schroeder MA, Hari PN, Blithe A, Paranagama D, Bhatt V, DiPersio JF. Safety analysis of patients who received ruxolitinib for steroid-refractory acute or chronic graft-versus-host disease in an expanded access program. Bone Marrow Transplant. 2022;57(6):975-981.
doi pubmed - Escamilla-Gomez V, Garcia Gutierrez V, Alcalde-Mellado P, Astibia-Mahillo B, Chinea-Rodriguez A, Lopez-Corral L, Acera-Gomez M, et al. Ruxolitinib in acute and chronic graft-versus-host disease: real life long-term experience in a multi-center study for adult and pediatric patients, on behalf of the GETH-TC. Bone Marrow Transplant. 2025;60(3):353-362.
doi pubmed - Escamilla Gomez V, Garcia-Gutierrez V, Lopez Corral L, Garcia Cadenas I, Perez Martinez A, Marquez Malaver FJ, Caballero-Velazquez T, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55(3):641-648.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.