| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 12, December 2025, pages 708-715
Plasma Level of Stromal Cell-Derived Factor-1 Provides Insight Into Statin Response, But Not Into Left Ventricular Dysfunction in New Unstable Angina
Hussam Murada, c, Mohammed Qutubb
aDepartment of Pharmacology, Faculty of Medicine, Rabigh Campus, King Abdulaziz University, Jeddah, Saudi Arabia
bDepartment of Cardiology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
cCorresponding Author: Hussam Aly Sayed Murad, Department of Pharmacology, Faculty of Medicine, Rabigh Campus, King Abdulaziz University, Jeddah 21589, Saudi Arabia
Manuscript submitted September 19, 2025, accepted December 12, 2025, published online December 24, 2025
Short title: Plasma SDF-1 and Statins in New UA
doi: https://doi.org/10.14740/jocmr6393
| Abstract | ▴Top |
Background: Stromal cell-derived factor-1 (SDF-1) is a chemokine that regulates atherogenesis, angiogenesis, and multiple physiological processes. Dyslipidemia can contribute to low plasma SDF-1 disturbing its vascular repair functions and elevating cardiovascular risk. Statin therapy is recommended for patients with acute coronary syndrome irrespective of low-density lipoprotein-cholesterol (LDL-C) levels. This study aimed to evaluate the potential associations of plasma SDF-1 levels with LDL-C levels, coronary occlusion-based disease severity, low left ventricular ejection fraction (LVEF) values, and statin therapy in patients with unstable angina (UA).
Methods: Patients with new UA (n = 108) were selected from Coronary Care Unit, King Abdulaziz University Hospital. The exclusion criteria included previous history of myocardial infarction, cardiac valvular problems, myocarditis, liver dysfunction, and recent acute infection. The demographic and clinical features were collected. Disease severity and LVEF values were determined. Plasma SDF-1, LDL-C, and troponin levels were measured.
Results: There were poor correlations between plasma SDF-1 level, and sociodemographic features and risk factors except with LDL-C, where it showed a significant correlation. Furthermore, plasma SDF-1 showed non-significant variations with LVEF values and troponin peak levels. In contrast, plasma SDF-1 declined significantly in statin-treated patients, regardless of LDL-C level, compared with those untreated. The receiver operating characteristic (ROC) curve for SDF-1 test showed good accuracy.
Conclusion: In patients with severe UA, plasma SDF-1 level showed significant variations with LDL-C levels and statin therapy suggesting that it can give insight into response to statin therapy regardless of LDL-C level. The ROC analysis showed favorable characteristics suggesting a potential usefulness of plasma SDF-1 assay to discriminate the statin-treated patients from those untreated. This novel approach highlights SDF-1 potential as a biomarker for monitoring statin therapy and predicting risks for adverse events in UA. Furthermore, it could pave the way for longitudinal studies with repeated measurements of plasma SDF-1 to evaluate its role as a prognostic indicator for major adverse cardiovascular events besides the other cardiovascular disease risk factors.
Keywords: Statins; Stromal cell-derived factor-1; Unstable angina
| Introduction | ▴Top |
The cardiovascular disease (CVD) related deaths have greatly increased, and the coronary artery disease (CAD) is “the Epidemic of the 20th Century” [1]. The acute coronary syndrome (ACS), which involves myocardial infarction (MI) and unstable angina (UA), is a life-threatening condition [2]. The extent of coronary plaque burden and stenosis severity are major predictors of adverse future events, alongside left ventricular ejection fraction (LVEF) [3]. Statins have anti-dyslipidemic and also nonlipid “pleiotropic” effects which strongly support their use for ACS treatment irrespective of blood cholesterol levels [4, 5].
The stromal cell-derived factor-1 (SDF-1; also known as CXCL12) is a chemokine expressed in different tissues. It regulates atherogenesis, angiogenesis, and multiple physiological processes [6]. The SDF1/CXCR4/CXCR7 axis plays a great role in cardiac ischemia and there is an association of certain CXCL12 gene polymorphisms with CAD in Asian populations [7]. The anti-thrombotic CXCL12/CXCR7 axis, through controlling platelet biogenesis and thrombo-inflammatory functions, antagonizes the pro-thrombotic CXCL12/CXCR4 pathway [8]. In patients with CAD, plasma CXCL12 level is an independent predictor of adverse CV events, and it improves risk reclassification [9]. Moreover, the higher plasma CXCL12 level is associated with elevated incidence of major adverse cardiovascular events (MACEs), and hence it could act as a prognostic indicator besides the other CAD risk factors [10]. The circulating SDF-1 was found significantly lower in patients with heterozygous familial hypercholesterolemia compared to healthy controls. It inversely correlated with plasma cholesterol, triglycerides, low-density lipoprotein-cholesterol (LDL-C), and oxidized LDL. These correlations highlight the potential common pathways between SDF-1 and lipoprotein metabolism enhancing its role in atherogenesis [11]. In these patients, dyslipidaemia, especially the high oxidized LDL, can contribute to low serum SDF-1 which may disturb the angiogenic and vascular repair functions elevating CV risk [12]. Decreasing LDL-C levels reduces ischemic stroke risk, but there still is a residual CV risk. Adopting a personalized medicine approach in lipid management to target LDL-C, remnant cholesterol, and triglycerides could effectively decrease the risk of carotid plaque rupture and cerebrovascular events, especially in female patients and patients below 70 years old [13].
Collectively, this work was designed to detect any potential associations between plasma level of SDF-1 and coronary occlusion-based disease severity, LVEF grades, LDL-C levels, and statin therapy in patients with new UA.
| Materials and Methods | ▴Top |
Subjects and study design
This study was approved by King Abdulaziz University (KAU)-Research Ethics Committee (No. 578-20) and was performed in agreement with the Helsinki Declaration. It involved 108 consecutive adult patients with new UA attack presenting for the first time to the Coronary Care Unit at KAU Hospital whether statin-treated or -untreated from December 2020 till November 2021.
UA was determined by transient cardiac ischemia resulting in decreased blood flow in the absence of significant myocardial necrosis detected by plasma troponin level [14]. The inclusion criteria were diagnosis of UA based on chest pain, ST segment depression, absence of elevated cardiac enzymes, and coronary angiography data according to the American Heart Association guidelines [15]. The exclusion criteria included previous history of MI, cardiac valvular problems, myocarditis, liver dysfunction, and recent acute infection. The demographic and clinical features such as age, gender, height, weight, body mass index, smoking, dyslipidemia, hypertension, diabetes mellitus, and statin therapy were collected. Patients were receiving atorvastatin 40 mg or rosuvastatin 20 mg daily irrespective of LDL-C level. Blood samples were taken to measure SDF-1, LDL-C, and troponin levels.
Evaluation of artery stenosis severity
The severity was evaluated during coronary angiography and was graded as 1-25%, 26-50%, 51-75%, or 76-100% [16]. The severity of the disease depends on number of the coronary arteries affected and degree of occlusion where: 1) mild: 1 - 2 coronary arteries blocked < 30%, 2) moderate: 1 - 2 blocked 30-49% or three blocked < 30%, 3) severe: 1 - 2 blocked ≥ 50%, or three blocked 30-49%, and 4) very severe: three or more blocked ≥ 50% [17]. Moreover, patients were diagnosed with severe UA if they meet one or more of the following features: rest angina > 20 min, recurrent episodes within 1 - 2 days, and elevated cardiac troponin but still below the MI diagnostic level [18].
LVEF grades
It was measured by the echocardiography and was expressed as normal (55-70%) or below normal (slight, moderate, and severe impairment) equivalent to 40-54%, 35-39%, and < 35%, respectively [19, 20].
Assay of plasma SDF-1 and LDL-C levels
The human ELISA kits (CXCL12/SDF-1 alpha, Quantikine DSA00, R&D system, MPLS, USA, and LDL-C, MBS752437, CA, USA) were used according to the manufacturer’s instructions. Full validation data of the SDF-1 ELISA kit including specificity, sensitivity, linearity, and precision were provided by the manufacturer. Moreover, a previous study that aimed to validate the kit, concluded that it is “a reproducible and valid assay for use with equine serum, plasma, and synovial fluid” [21]. To confirm reliable performance in our lab, a calibration curve was constructed from the provided standard for linear regression. Each standard dilution was read twice, and the coefficients of variation (CV) were calculated (Table 1). The CV% values are acceptable (< 15%) [22], and the coefficient of determination (R2) is equal to 0.990, indicating a strong positive linear relationship with the model predicting the outcome with a high degree of accuracy (Fig. 1). All samples were measured in duplicate according to the manufacturer’s instructions.
 Click to view | Table 1. Data for SDF-1 Calibration Curve: Duplicate Optical Density Readings (R1 and R2) for Blank and Standard Serial Dilutions (S1-S6) |
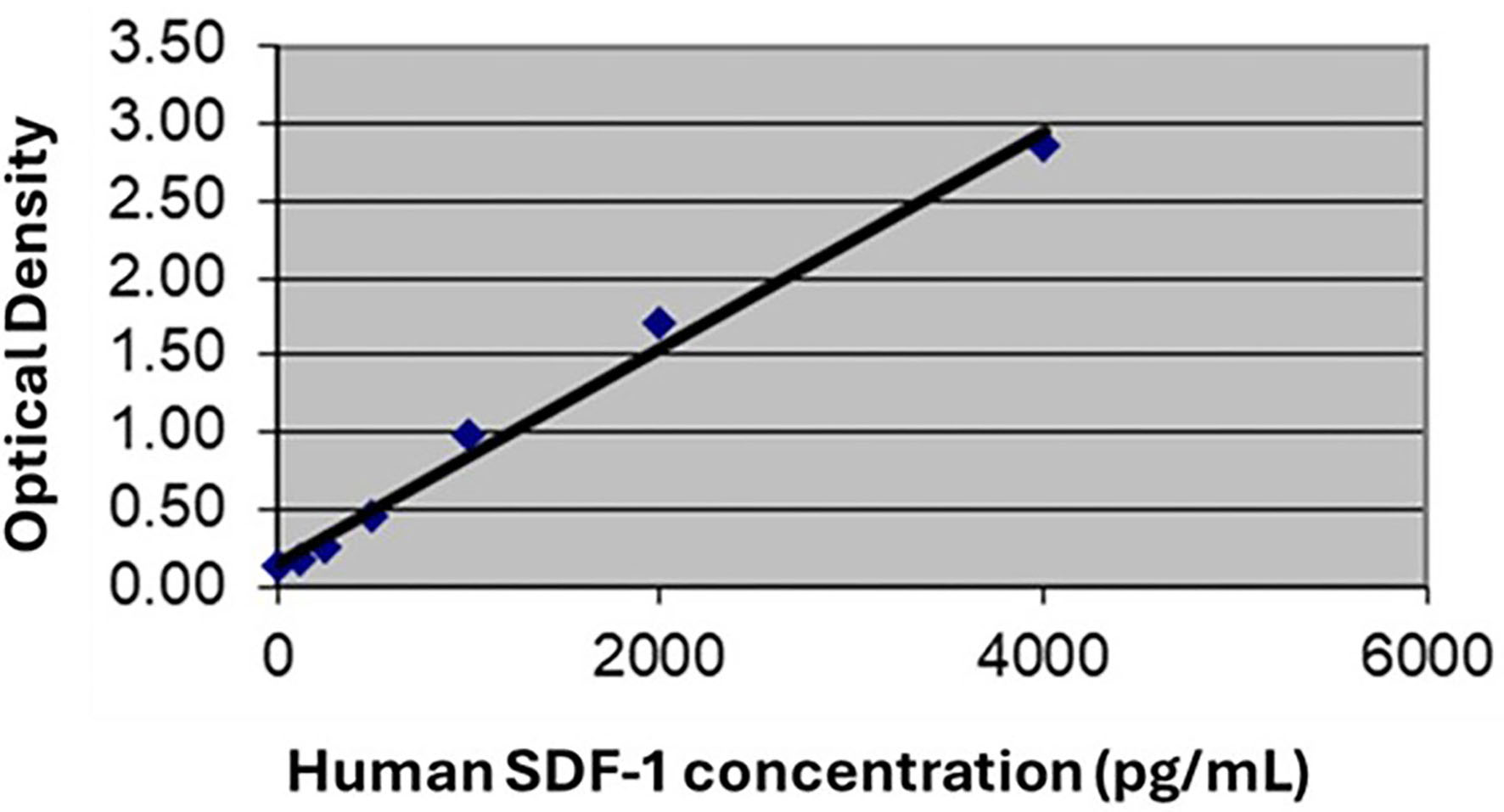 Click for large image | Figure 1. Calibration curve for SDF-1 assay. The coefficient of determination (R2) is 0.990, indicating a strong positive linear relationship and a high accuracy in predicting the outcome. SDF-1: stromal cell-derived factor-1. |
Troponin assay
Plasma cardiac troponin I level was measured by the Immulite turbo-troponin I (DPC method, LA, USA) at admission and at 8, 12, 18, and 24 h following start of symptoms to detect its maximum value [23]. Its normal level is 0.04 to < 0.12 ng/mL, and the elevation may be mild, moderate, severe, or very severe equivalent to 0.12 to < 10, 10 to < 50, 50 to < 100, and ≥ 100 ng/mL, respectively.
Statistical analysis
Data were analyzed using SPSS software (version 22, IL, USA). Quantitative variables were presented as mean ± standard error of the mean (SEM), while categorical variables were expressed as frequencies and percentages. The analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used for comparing the patients and control groups. The sample size was calculated using an online tool with a significance level of 0.05, a test power of 0.8, minimum detectable effect of 0.25, and a standard deviation (SD) of 0.926. In the association studies, 80% as a statistical power is widely used to avoid false-negative associations and to calculate a cost-effective sample size [24]. The linear regression analysis was conducted to detect associations between plasma SDF-1 level (dependent variable) and sociodemographic and risk factors (predictor variables). Spearman’s correlation coefficient was used to detect associations between SDF-1 levels, LVEF grades, statin therapy, and troponin peaks. The receiver operating characteristic (ROC) curve was generated to quantify the precision of the SDF-1 level test for differentiating statin-treated from statin-untreated patients. The closer the ROC curve is to the upper left point, the higher the overall accuracy of the test [25]. P values < 0.05 were considered significant.
| Results | ▴Top |
Degree of severity of UA
The UA severity was assessed according to number of coronary arteries affected and degree of occlusion (Table 2) and most cases were in the severe category.
 Click to view | Table 2. Degree of Severity of UA Based on Number of Coronary Arteries Affected and Degree of Occlusion Expressed as Frequencies and Percentages |
Levels of CXCL12 and its relationship with sociodemographic features, risk factors, and comorbidities in statin-treated and -untreated patients with severe UA
In patients with severe UA, plasma CXCL12 level was 2191.30 ± 205.98 pg/mL. In Table 3, the linear regression analysis between sociodemographic features, risk factors, and associated comorbidities (predictor variables), and plasma CXCL12 level (dependent variable) showed small non-significant values of the coefficient of determination (R2) in statin and no-statin groups indicating absent or poor correlation. Exceptionally, R2 value with LDL categories was significant (P < 0.05). Optimal LDL-C level was < 100 mg/dL [26].
 Click to view | Table 3. Coefficient of Determination (R2) Values of Linear Regression Analysis Between Sociodemographic Features, Risk Factors, and Associated Comorbidities (Predictor Variables), and Plasma SDF-1 Levels (pg/mL) (Dependent Variable) in Severe UA Patients (n = 61) |
Relationships between plasma CXCL12 levels, and LVEF, troponin peak levels, statin therapy, and antiplatelet therapy in patients with severe UA
The plasma CXCL12 levels showed non-significant variations with LVEF grades, troponin peak levels, and type of antiplatelet drug used. In contrast, plasma SDF-1 levels declined significantly (P = 0.001) in statin-treated patients compared with those untreated (Table 4). Furthermore, Spearman’s correlation showed a significant inverse correlation between SDF-1 levels and the statin treatment (P < 0.001). The area under the ROC curve (AUROC) was calculated to quantify precision of CXCL12 assay in discriminating statin-treated from -untreated patients. The AUROC for CXCL12 (Fig. 2) was 0.791 with a significant P value (< 0.001), and the sensitivity and specificity values were 79.3% and 71.9% at a concentration (cutoff) of 1,954 pg/mL.
 Click to view | Table 4. Plasma Levels of SDF-1 (pg/mL) in the Different Age Groups, Gender Categories, Left Ventricular Ejection Fraction (LVEF) Categories, Troponin Peak Levels, Statin Therapy Groups, and Antiplatelet Therapy Groups in Severe UA Patients (n = 61) |
 Click for large image | Figure 2. Receiver operating characteristic (ROC) curve for SDF-1 test in statin-treated and -untreated patients. The curve (blue line) is closer to the upper left corner indicating high accuracy of differentiation between the two groups. The area under the ROC curve (AUC) = 0.791, 95% confidence interval (95% CI) = 0.679 - 0.903; and P value < 0.001. SDF-1: stromal cell-derived factor-1. |
| Discussion | ▴Top |
The common risk factors and comorbidities associated with CAD include dyslipidemia, hypertension, diabetes, and smoking [27, 28]. The current results showed that in patients with severe new UA, there were poor correlations between the plasma CXCL12 level, and sociodemographic features, risk factors, and comorbidities except with LDL categories, where it showed a significant correlation. Furthermore, plasma CXCL12 levels declined significantly in statin-treated patients regardless of LDL-C level compared with those untreated. In contrast, they showed non-significant variations with LVEF and troponin peak levels. The SDF-1 levels demonstrated a significant inverse correlation with the statin treatment, while showed a non-significant variation with the type of antiplatelet drug used. The ROC curve analysis for SDF-1 assay indicated good overall diagnostic accuracy of the test to discriminate between statin-treated and -untreated patients. The optimal cutoff was derived by using the “Minimal distance to the perfect test point (0,1)” at the upper left corner in the ROC curve. The minimal distance approach is a well-established and accepted method in diagnostic test evaluation. It balances both sensitivity and specificity to identify the cutoff that is closest to optimal diagnostic performance. Furthermore, it is an appropriate method when the threshold range is wide, as is the case in our model.
In the current study, we focused on UA severe cases because our sample size is relatively small, and only the number of severe UA cases is sufficient for analysis, while the numbers of mild, moderate, and very severe cases are not. Also, concentrating on severe UA cases ensured a more uniform study population, strengthening the reliability of analysis. Moreover, patients with severe UA face the greatest risk of adverse outcomes, so identifying new markers to predict response to treatment and potential complications is clinically meaningful.
Previously, it was reported that in UA patients, CXCL12 has anti-atherogenic properties due to its anti-inflammatory and matrix-stabilizing effects especially in high concentrations, and therefore enhancing CXCL12 activity could be potential therapy. Plasma CXCL12 levels in UA patients were lower compared with those stable angina patients and healthy controls [29]. In contrast, another study reported that CXCL12 expression on platelets in patients with stable angina was higher than healthy controls suggesting a pro-thrombotic role [30]. In ACS patients following vascular injury, platelet-derived CXCL12 expression occurs faster than other biomarkers like troponin-I, suggesting that CXCL12 could be a useful early biomarker of vascular injury [31]. In MI patients, serum CXCL12 level significantly correlated with severity of coronary obstruction up to a certain limit of occlusion, after which there was no significant variation [32]. Additionally, plasma SDF-1 levels strongly correlated with ACS severity judged by number of coronaries affected and degree of occlusion [33]. Moreover, it was previously reported that CAD patients with elevated SDF-1 levels more probably had left ventricular impairment with ejection fraction < 45% and that high plasma SDF-1 level is an independent predictor of adverse CV outcomes [9].
The guidelines recommend using fixed dose statins instead of focusing on specific LDL targets; however, the on-treatment achieved LDL level was reported to be a significant predictor of MACE [34]. In CAD patients, statins decrease CV morbidity and mortality, even in patients having ideal LDL-C levels (< 100 mg/dL) [26]. In patients with recent ACS, intensive statin therapy lowered LDL levels and reduced MACE for 2 years [35]. A previous study reported that statins strongly and dose-dependently reduced CXCL12 levels. High doses of statins could stabilize the atherosclerotic plaque by inhibiting angiogenesis within the plaque and/or neovascularization in the ischemic myocardium [36]. Moreover, subjects having higher CXCL12 levels were less likely to have dyslipidemia and to be on statins at the time of enrolment [9]. Furthermore, statins change cholesterol crystallization in arteries providing stabilization of the atherosclerotic plaque [37]. In recent clinical studies, statins have been proved to reduce the percentage of atherosclerotic plaque volume and total volume [38]. Lipid-lowering agents have been reported to reduce coronary plaque volume, enhance plaque stability, and minimize the occurrence of adverse clinical events. However, the prolonged safety of very low LDL-C levels needs to be investigated by clinical trials [39]. The decreased SDF-1 levels under statin therapy might be significant because this could potentially reflect altered endothelial repair or regenerative activity. Several studies, using the recent invasive and non-invasive imaging techniques, have shown that statins exert direct effects (beyond decreasing LDL-C) on the atherosclerotic plaque morphology and composition enhancing its stabilization and regression, hence improving the clinical outcomes [40].
In the current study, cardiac troponin peaks were not elevated in UA patients, and the correlations between LVEF and troponin peak levels were non-significant. In ACS patients, it was reported that the ventricular systolic function is the best predictor of mortality, and that cardiac troponin is a useful diagnostic and prognostic tool of myocardial damage. Patients with MI having elevated troponin levels are more prone to adverse events than UA patients where troponin levels are normal [41]. Currently, many physicians use a single troponin test which appears to be as safe and accurate as serial troponin tests for selected groups of patients. This diagnostic approach is useful to decrease costs and relieve hospital crowding [42].
The single-center design and relatively small number of patients limit the generalizability of the current results. Therefore, for further evaluation of the relations between SDF-1 level, LVEF categories, and MACE, a longitudinal observational study with repeated measurements of plasma CXCL12 level and collection of follow-up data is recommended. Another limitation is lack of evaluation of the correlation between plasma SDF-1 level and LV global longitudinal strain.
In conclusion, in patients with severe UA (having 1 - 2 coronary arteries blocked ≥ 50% or three coronaries blocked 30-49%), plasma SDF-1 level showed significant variations with the LDL-C levels and statin therapy, indicating that it can provide insight into response to statin therapy regardless of LDL-C level. In contrast, SDF-1 level non-significantly correlated with LVEF, and troponin peak levels. The ROC curve for CXCL12 test showed favorable characteristics suggesting a potential use as a marker to discriminate between statin-treated from statin-untreated patients. Further evaluation of the relations between SDF-1 levels, LVEF grades, and MACE in a longitudinal observational study with repeated measurements of plasma CXCL12 level is recommended.
Acknowledgments
None to declare.
Financial Disclosure
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia under grant no. IPP: 471-828-2025. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consents were obtained from all participants in the study.
Author Contributions
HM conceived the idea, designed the work, and wrote the first draft of the manuscript. MQ determined UA and evaluated the disease severity. HM and MQ conducted the research and collected and analyzed data. They revised and approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACS: acute coronary syndrome; AUROC: area under receiver operating characteristic curve; BMI: body mass index; CV: coefficient of variation; CAD: coronary artery disease; CVD: cardiovascular disease; CXCL12: chemokine (C-X-C motif) ligand 12; LDL-C: low-density lipoprotein-cholesterol; LVEF: left ventricular ejection fraction; MACEs: major adverse cardiovascular events; MI: myocardial infarction; R2: coefficient of determination; SDF-1: stromal cell-derived factor-1; UA: unstable angina
| References | ▴Top |
- Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014;127(9):807-812.
doi pubmed - Daga LC, Kaul U, Mansoor A. Approach to STEMI and NSTEMI. J Assoc Physicians India. 2011;59 Suppl:19-25.
pubmed - Vatsa N, Faaborg-Andersen C, Dong T, Blaha MJ, Shaw LJ, Quintana RA. Coronary atherosclerotic plaque burden assessment by computed tomography and its clinical implications. Circ Cardiovasc Imaging. 2024;17(8):e016443.
doi pubmed pmc - Ostadal P. Statins as first-line therapy for acute coronary syndrome? Exp Clin Cardiol. 2012;17(4):227-236.
pubmed pmc - Chou R, Dana T, Blazina I, Daeges M, Bougatsos C, Grusing S, Jeanne TL. Statin use for the prevention of cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force [internet]. Report No.: 14-05206-EF-2. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016.
pubmed pmc - Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34(4):742-750.
doi pubmed - Zhang J, Ma H, Gao J, Kong S, You J, Sheng Y. Variants in the CXCL12 gene was associated with coronary artery disease susceptibility in Chinese Han population. Oncotarget. 2017;8(33):54518-54527.
doi pubmed pmc - Chatterjee M. Atypical roles of the chemokine receptor ACKR3/CXCR7 in platelet pathophysiology. Cells. 2022;11(2):213.
doi pubmed pmc - Ghasemzadeh N, Hritani AW, De Staercke C, Eapen DJ, Veledar E, Al Kassem H, Khayata M, et al. Plasma stromal cell-derived factor 1alpha/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis. 2015;238(1):113-118.
doi pubmed pmc - Zhang S, Ding Y, Feng F, Gao Y. The role of blood CXCL12 level in prognosis of coronary artery disease: a meta-analysis. Front Cardiovasc Med. 2022;9:938540.
doi pubmed pmc - Juhasz L, Lorincz H, Szentpeteri A, Toth N, Varga E, Paragh G, Harangi M. Decreased serum stromal cell-derived factor-1 in patients with familial hypercholesterolemia and its strong correlation with lipoprotein subfractions. Int J Mol Sci. 2023;24(20):15308.
doi pubmed pmc - Juhász L, Lőrincz H, Szentpéteri A, Tóth N, Varga É, Paragh G, Harangi M, et al. Assessment of serum stromal cell-derived factor-1 in patients with familial hypercholesterolemia and its correlation with lipoprotein subfractions. Atherosclerosis. 2024;395(Supplement 1):118012.
- Servadei F, Scimeca M, Palumbo V, Oddi FM, Bonfiglio R, Giacobbi E, Menghini R, et al. Aging and sex modify the risk of carotid plaque thrombosis related to dyslipidemic profile. Stroke. 2025;56(10):2879-2887.
doi pubmed pmc - Rao SV, O'Donoghue ML, Ruel M, Rab T, Tamis-Holland JE, Alexander JH, Baber U, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2025;85(22):2135-2237.
doi pubmed - Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239.
doi pubmed - Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99(17):2345-2357.
doi pubmed - Johns Hopkins Medicine. Updated classification system captures many more people at risk for heart attack. https://www.hopkinsmedicine.org/news/media/releases/updated_classification_system_captures_many_more_people_at_risk_for_heart_attack. 2017 (Accessed March 6, 2022).
- Hamm CW, Braunwald E. A classification of unstable angina revisited. Circulation. 2000;102(1):118-122.
doi pubmed - Silbiger JJ. Pathophysiology and echocardiographic diagnosis of left ventricular diastolic dysfunction. J Am Soc Echocardiogr. 2019;32(2):216-232.e212.
doi pubmed - Ramchand J, Podugu P, Obuchowski N, Harb SC, Chetrit M, Milinovich A, Griffin B, et al. Novel approach to risk stratification in left ventricular non-compaction using a combined cardiac imaging and plasma biomarker approach. J Am Heart Assoc. 2021;10(8):e019209.
doi pubmed pmc - Brown MP, Dymock DC, Merritt KA, Trumble TN. Stromal cell-derived factor-1 (SDF-1) validation using equine serum, plasma, and synovial fluid. Osteoarthr Cartil. 2014;22:S71.
- Uvarova NE, Eremenko NN, Ramenskaya GV, Goryachev DV, Smirnov VV. Comparison of FDA (2018) and EAEU regulatory requirements for bioanalytical method validation. Pharm Chem J. 2019;53:759-765.
- Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124(21):2350-2354.
doi pubmed - Ahn C. Sample size and power estimation in case-control genetic association studies. Genomics Inform. 2006:4(2):51-56.
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-577.
pubmed - Arnold SV, Spertus JA, Tang F, Krumholz HM, Borden WB, Farmer SA, Ting HH, et al. Statin use in outpatients with obstructive coronary artery disease. Circulation. 2011;124(22):2405-2410.
doi pubmed pmc - Salehi R, Motemavele M, Goldust M. Risk factors of coronary artery disease in women. Pak J Biol Sci. 2013;16(4):195-197.
doi pubmed - Takieddin SZ, Alghamdi NM, Mahrous MS, Alamri BM, Bafakeeh QA, Zahrani MA. Demographics and characteristics of patients admitted with acute coronary syndrome to the Coronary Care Unit at King Abdulaziz University. Cureus. 2022;14(6):e26113.
doi pubmed pmc - Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, Holm AM, et al. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106(1):36-42.
doi pubmed - Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kogel A, Pfaff F, et al. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30(5):584-593.
doi pubmed - Wurster T, Stellos K, Haap M, Seizer P, Geisler T, Otton J, Indermuehle A, et al. Platelet expression of stromal-cell-derived factor-1 (SDF-1): an indicator for ACS? Int J Cardiol. 2013;164(1):111-115.
doi pubmed - Wyderka R, Wojakowski W, Jadczyk T, Maslankiewicz K, Parma Z, Pawlowski T, Musialek P, et al. Mobilization of CD34+CXCR4+ stem/progenitor cells and the parameters of left ventricular function and remodeling in 1-year follow-up of patients with acute myocardial infarction. Mediators Inflamm. 2012;2012:564027.
doi pubmed pmc - Tavakolian Ferdousie V, Mohammadi M, Hassanshahi G, Khorramdelazad H, Khanamani Falahati-Pour S, Mirzaei M, Allah Tavakoli M, et al. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int J Cardiol. 2017;233:23-28.
doi pubmed - Ross EG, Shah N, Leeper N. Statin intensity or achieved LDL? Practice-based evidence for the evaluation of new cholesterol treatment guidelines. PLoS One. 2016;11(5):e0154952.
doi pubmed pmc - Yu S, Jin J, Chen Z, Luo X. High-intensity statin therapy yields better outcomes in acute coronary syndrome patients: a meta-analysis involving 26,497 patients. Lipids Health Dis. 2020;19(1):194.
doi pubmed pmc - Camnitz W, Burdick MD, Strieter RM, Mehrad B, Keeley EC. Dose-dependent effect of statin therapy on circulating CXCL12 levels in patients with hyperlipidemia. Clin Transl Med. 2012;1(1):23.
doi pubmed pmc - Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am J Cardiol. 2011;107(12):1710-1717.
doi pubmed - Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, Gadhoke NV, et al. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis. 2020;306:85-95.
doi pubmed - Zhang X, Feng H, Han Y, Yuan X, Jiang M, Wang W, Gao L. Plaque stabilization and regression, from mechanisms to surveillance and clinical strategies. Rev Cardiovasc Med. 2024;25(12):459.
doi pubmed pmc - Papafaklis MI, Koros R, Tsigkas G, Karanasos A, Moulias A, Davlouros P. Reversal of atherosclerotic plaque growth and vulnerability: effects of lipid-modifying and anti-inflammatory therapeutic agents. Biomedicines. 2024;12(11):2435.
doi pubmed pmc - Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, Plein S, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017;12(2):147-155.
doi pubmed pmc - Wassie M, Lee MS, Sun BC, Wu YL, Baecker AS, Redberg RF, Ferencik M, et al. Single vs serial measurements of cardiac troponin level in the evaluation of patients in the emergency department with suspected acute myocardial infarction. JAMA Netw Open. 2021;4(2):e2037930.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.