Prevalence of Alloimmunization Events in Thalassemia Patients With Repeated Transfusions in the Rhesus Blood Group System: A Systematic Review and Meta Analysis
DOI:
https://doi.org/10.14740/jocmr6142Keywords:
Alloimmunization, Antibodies, Rhesus, Thalassemia, TransfusionAbstract
Background: Alloimmunization presents a significant challenge for patients with β-thalassemia major who depend on regular transfusion therapy. This systematic review and meta-analysis aimed to evaluate the frequency of alloimmunization within the Rhesus blood group system and identify the most prevalent alloantibodies.
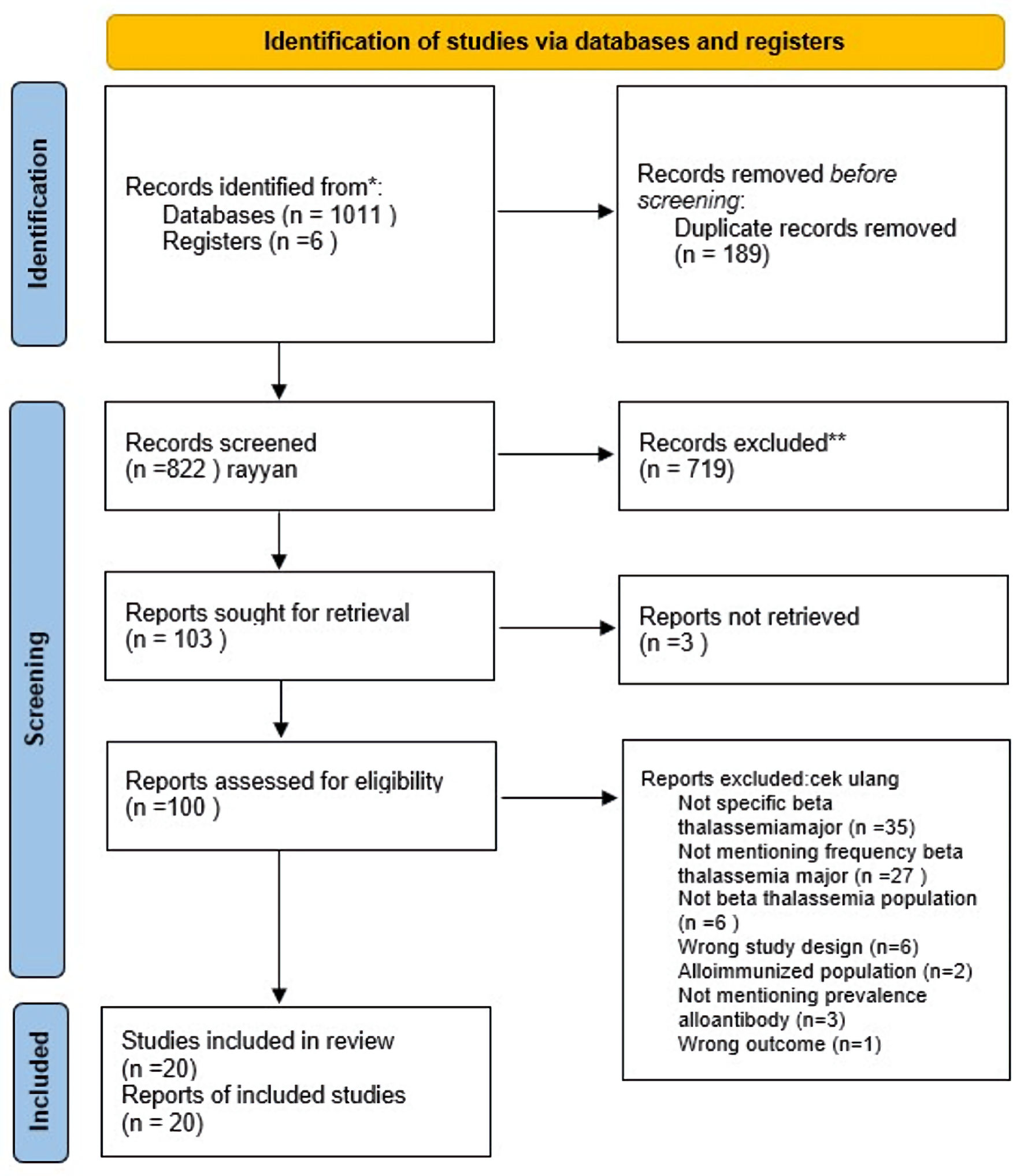
Methods: A comprehensive search across multiple databases was conducted to locate epidemiological studies reporting alloimmunization in thalassemia patients undergoing repeated transfusions, specifically focusing on Rhesus antibodies. Statistical analyses were performed using R software, and heterogeneity was assessed using I2 statistics.
Results: This review included 20 studies with a total of 4,650 patients. The overall prevalence of alloimmunization was 5.4% (95% confidence interval (CI): 3.1-9.3%) across all ages, with a prevalence of 9.1% (95% CI: 5.3-15.2%) in children and 25% (95% CI: 12.7-41.2%) in adults. The pooled overall prevalence was 6.6% (95% CI: 4.2-10.2%). Among the 488 alloimmunized patients, 310 developed Rhesus-specific antibodies, with anti-E (34.58%) and anti-D (13.69%) being the most frequent.
Conclusions: This study underscores the substantial prevalence of Rhesus antibodies among alloimmunized thalassemia patients. Implementing extended phenotype matching for transfusions could significantly reduce the risk of alloantibody formation in this population. Future analyses should explore factors influencing alloimmunization rates, such as ethnic diversity, matching protocols, and age-related variations, to inform clinical practice better.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








