Safety of Molnupiravir in Hospitalized Patients With Coronavirus Disease 2019: A Retrospective, Single-Center, Cohort Study
DOI:
https://doi.org/10.14740/jocmr6297Keywords:
Coronavirus disease 2019, Molnupiravir, Adverse events, Creatine phosphokinase, AnemiaAbstract
Background: Molnupiravir (MOV) is recommended for the treatment of patients with coronavirus disease 2019 (COVID-19) who are ineligible for remdesivir treatment. However, data regarding laboratory-based adverse events (AEs) associated with MOV use in hospitalized patients are limited. In this study, we evaluated MOV-associated laboratory abnormalities, including increased creatine phosphokinase (CPK) and decreased hemoglobin (Hb) levels (e.g., anemia), and evaluated related risk factors in hospitalized COVID-19 patients.
Methods: We reviewed retrospective data for 78 adult inpatients with COVID-19 who received MOV upon admission at Fukuoka University Chikushi Hospital. Individuals with MOV treatment history, early discontinuation, or premature discharge were excluded. Data were collected on demographics, Charlson Comorbidity Index, bacterial coinfection, concomitant medications, COVID-19 severity, oxygen therapy, and length of hospital stay. The AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Japanese Clinical Oncology Group. Multivariate analyses were conducted to identify risk factors for elevated CPK levels and anemia.
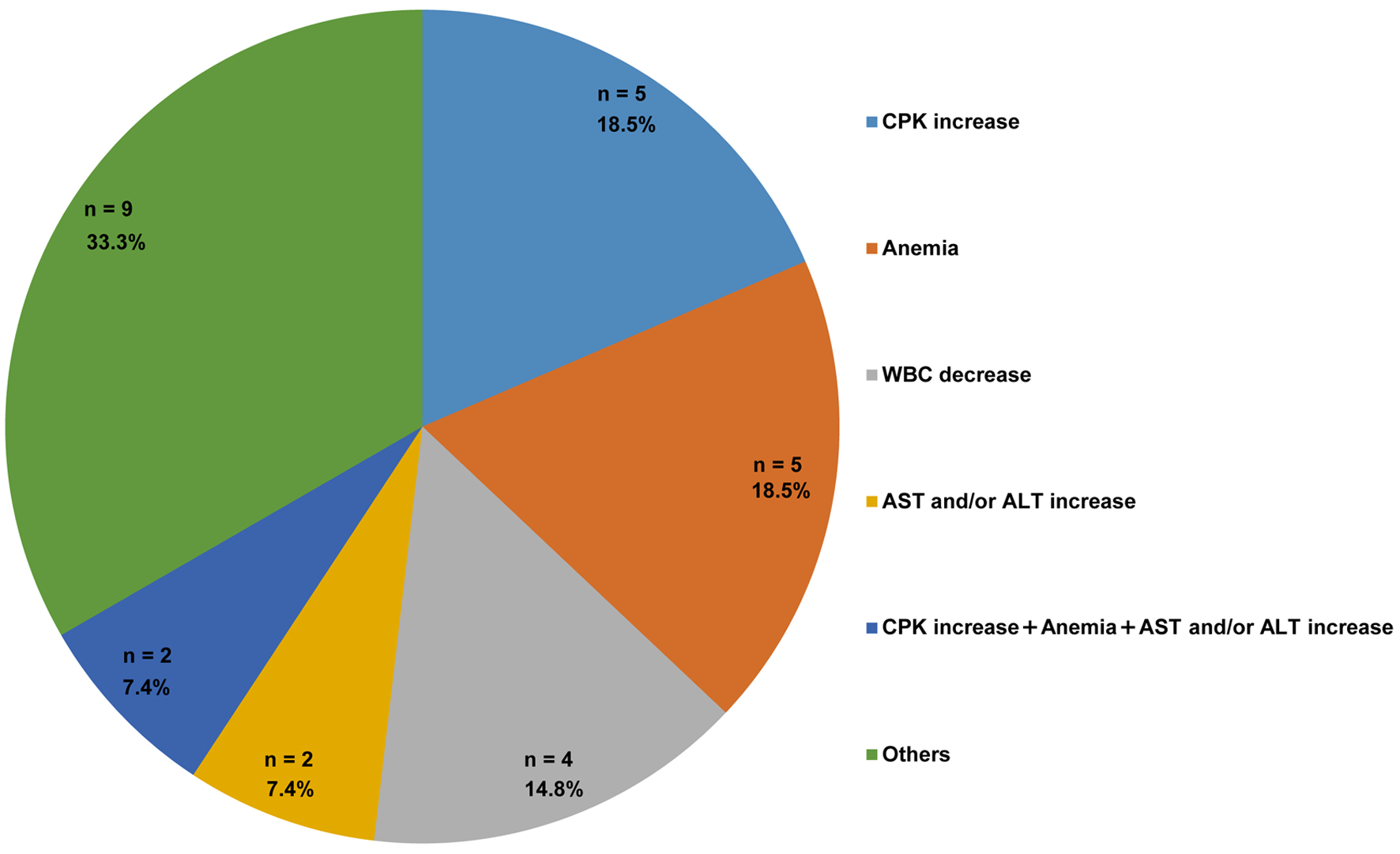
Results: The median age of the study population was 82 years (interquartile range: 74 - 89 years; men: 56.4%), and 17.9% had bacterial coinfection. Twenty-seven patients (34.6%) experienced ≥ 1 AE, and MOV was discontinued in two patients because of a mild rash and CPK elevation. Elevated CPK levels and anemia each occurred in 11 patients (14.1%). Severe AEs (grade ≥ 3) were observed in one patient with grade 4 CPK elevation and in another with grade 3 anemia. Multivariate analysis showed that bacterial coinfection tended to increase CPK levels (adjusted odds ratio (aOR): 3.30, 95% confidence interval (CI): 0.75 - 13.32, P = 0.10) and was significantly associated with anemia (aOR: 5.40, 95% CI: 1.27 - 23.69, P = 0.022).
Conclusions: MOV exhibits a generally favorable safety profile in hospitalized COVID-19 patients, with low treatment discontinuation rates and mild laboratory abnormalities. Elevated CPK levels and anemia may reflect the complications of bacterial coinfection rather than direct MOV toxicity; however, these results should be interpreted with caution because of the small sample size and single-center, non-controlled study design. Further multicenter prospective studies are warranted to determine the relationship between CPK elevation, anemia, and MOV treatment in COVID-19 patients.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








