Higher Processed Blood Volume of Granulocyte and Monocyte Adsorption Apheresis Ameliorates Long-Term Disease Activity in Ulcerative Colitis Patients
DOI:
https://doi.org/10.14740/jocmr6071Keywords:
Ulcerative colitis, Granulocyte and monocyte adsorption apheresis, Remission induction therapyAbstract
Background: Granulocyte and monocyte adsorption apheresis (GMA) is a therapeutic option for remission induction in the active ulcerative colitis (UC) patients. Effects of high processed blood volume of GMA as remission induction therapy on the long-term prognosis of UC patients have remained unclear. For this study, we investigated the relation between re-exacerbation of UC and the processed blood volume of GMA performed as induction therapy.
Methods: Data from UC patients treated using a total of 10 GMA sessions as remission induction therapy during 2012 - 2022 were retrospectively collected and analyzed. The relation between the GMA dose, processed blood volume of GMA divided by body weight, and UC re-exacerbation requiring inpatient treatment within 1 year was evaluated.
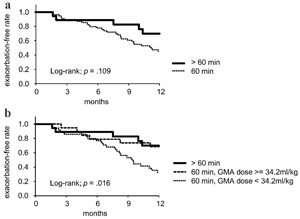
Results: This study examined data of 72 active UC patients, with median age of 44.4 years (65% male) and median GMA dose of 34.2 mL/kg/session. Kaplan-Meier analysis showed the 1-year exacerbation-free rate was significantly higher in the higher GMA dose group than in the lower GMA dose group (P = 0.008). Cox proportional hazards regression analyses revealed a higher GMA dose as inversely associated with the re-exacerbation of UC within 1 year (hazard ratio: 0.36, 95% confidence interval: 0.17 - 0.78). Extended treatment time of GMA session beyond 60 min contributed to achieving the higher GMA dose and did not increase unexpected treatment termination because of clotting.
Conclusion: Greater processed blood volume of GMA per patient body weight may be associated with a lower 1-year exacerbation rate in UC patients.
Published
Issue
Section
License
Copyright (c) 2024 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








