Comparative Efficacy of Tirzepatide vs. Semaglutide in Reducing Body Weight in Humans: A Systematic Review and Meta-Analysis of Clinical Trials and Real-World Data
DOI:
https://doi.org/10.14740/jocmr6231Keywords:
Semaglutide (Ozempic, Rybelsus, Wegovy), Tirzepatide (LY3298176, Zepbound, Mounjaro), Glucagon-like peptides, Body weight, Weight loss, Anti-obesity agentsAbstract
Background: The aim of the study was to compare the effectiveness of tirzepatide versus semaglutide in producing weight loss.
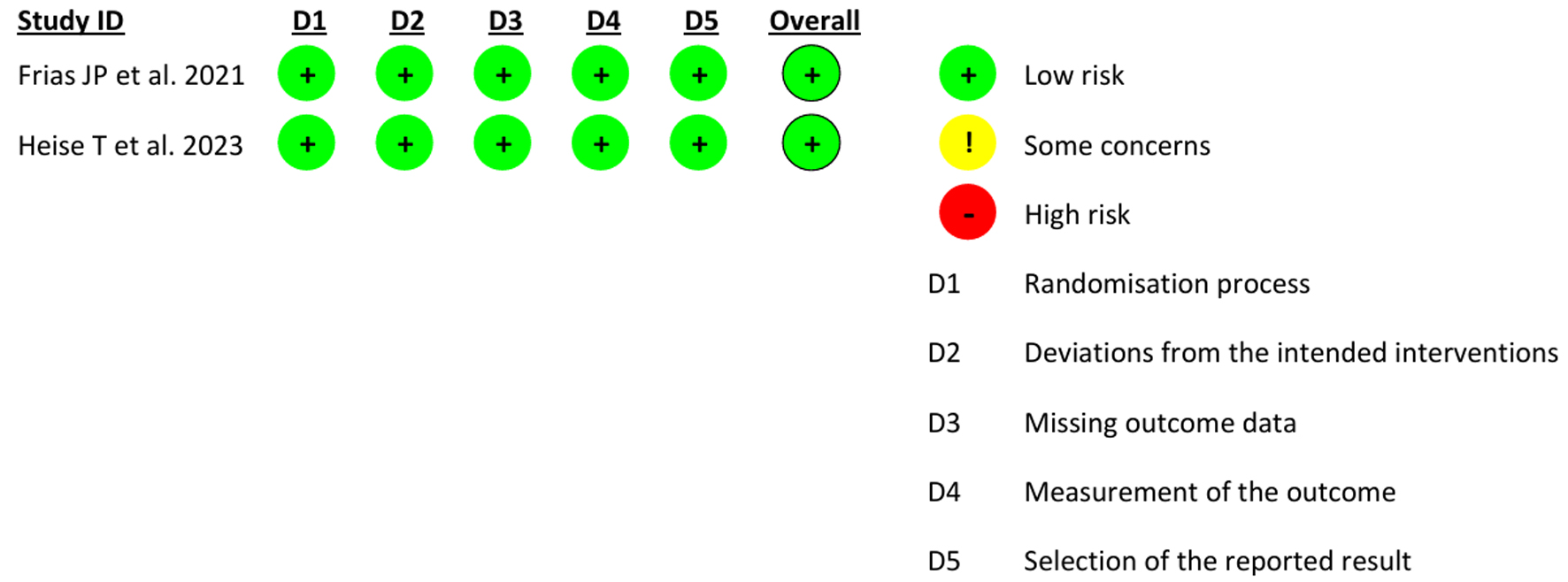
Methods: A systematic search was conducted in databases PubMed, Scopus, and Web of Science on January 22, 2025, using search terms (“tirzepatide,” “semaglutide,” and “weight loss”) and their alternatives, which yielded 751 studies in total. After deduplication, title/abstract and full text screening was conducted, and studies were assessed based on the eligibility criteria. After extracting the data, a meta-analysis (MA) was performed through RStudio. Heterogeneity among studies was evaluated with Cochran’s Q and I2 tests. A random-effect model was used to calculate pooled “mean differences” (MDs). Study quality was estimated by Newcastle-Ottawa Quality Assessment Scale (NOS) and Cochrane risk of bias (RoB) version 2 tool, and publication bias was estimated through forest plots and the Egger’s test.
Results: A total of two randomized controlled trials (RCTs) and five retrospective cohorts were included in this MA. MA results showed that compared with the semaglutide, tirzepatide could produce significantly greater weight loss (MD = 4.23; 95% confidence interval (CI): 3.22 - 5.25; P < 0.01). Subgroup analysis showed a dose- and duration-dependent significantly superior therapeutic effect of tirzepatide (> 10 mg dose: MD = 6.50, 95% CI: 5.93 - 7.08, P < 0.01 vs. ≤ 10 mg: MD = 3.89, 95% CI: 2.12 - 5.65, P < 0.01) (> 6 months duration: MD = 5.00, 95% CI: 3.48 - 6.52, P < 0.01 vs. ≤ 6 months: MD = 3.50, 95% CI: 2.24 - 4.75, P < 0.01). The supremacy of tirzepatide was maintained in both types of studies: RCTs and retrospective cohorts. No publication bias was found through forest plots visually or Egger’s test (Egger’s regression asymmetry test P value 0.94). Study quality estimated by NOS revealed the quality of each study as “good” (≥ 7 points) and that estimated by the Cochrane RoB tool revealed “low” RoB.
Conclusion: The pooled analysis provides evidence that tirzepatide is better than semaglutide in reducing body weight, regardless of study design. A dose-response relationship exists, and the weight loss magnitude increases with the dose or duration of tirzepatide. The studies that provide this evidence are of high quality and have a low RoB.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








