Molecular and Immune Microenvironmental Changes Across Endometrial Lesions: A Comprehensive Immunohistochemical and Clinical Analysis of Progression From Benignity to Carcinoma
DOI:
https://doi.org/10.14740/jocmr6342Keywords:
Endometriosis, Adenomyosis, Endometriosis-associated carcinoma, CD163, Endometrial hyperplasia, FOXP3, P16, CDK4, Cyclin D1Abstract
Background: Adenomyosis and endometriosis are estrogen-driven disorders with a recognized potential for malignant transformation, particularly through atypical endometriosis. The molecular and immune mechanisms underlying this progression remain incompletely understood. However, clinical factors such as age, comorbidities, and hormonal therapy can also influence lesion behavior. The objectives were to comprehensively evaluate hormonal, proliferative-apoptotic, cell-cycle, and immune-microenvironmental alterations across the spectrum of endometrial lesions and to assess the impact of prior endometrial hyperplasia and associated clinical parameters.
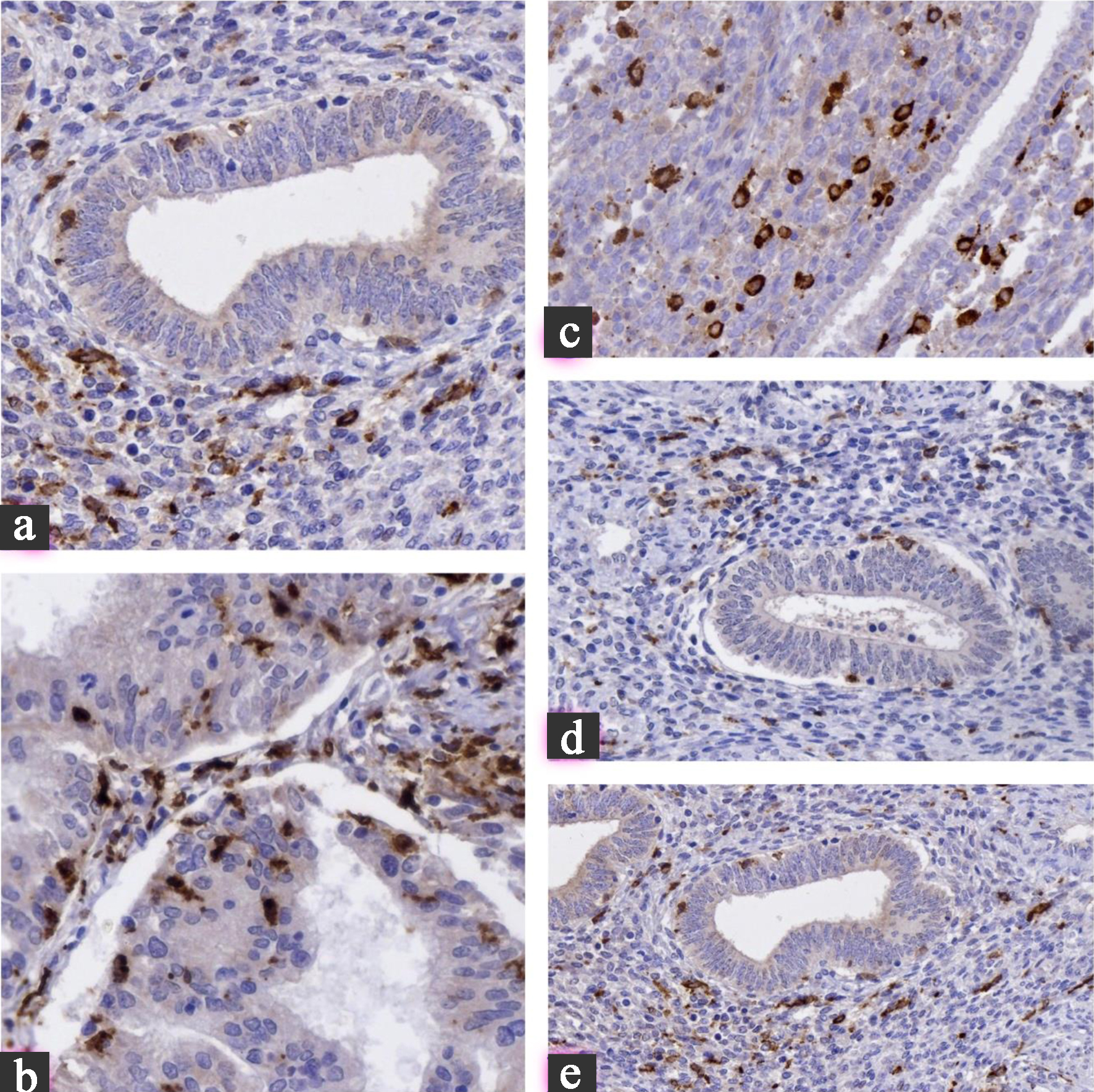
Methods: Seventy-seven formalin-fixed paraffin-embedded cases were stratified into five groups: eutopic endometrium (n = 17), adenomyosis (n = 27), typical endometriosis (n = 16), atypical endometriosis (n = 10), and endometriosis-associated carcinoma (n = 24). Immunohistochemical analysis included estrogen receptor, progesterone receptor, Ki67, BCL2, P53, cyclin D1, CDK4, P16, FOXP3, CD68, and CD163. Clinical variables including age, comorbidities, and medication history were integrated into statistical analysis. Marker expression was quantified semi-quantitatively, and clinical associations with prior endometrial hyperplasia were evaluated using Kruskal-Wallis and Mann-Whitney U tests.
Results: Cyclin D1, CDK4, and P16 expression progressively increased from benign lesions to carcinoma (P < 0.001). FOXP3+ T cells and CD163+ M2 macrophages accumulated in atypical endometriosis and carcinoma, indicating an immunosuppressive microenvironment. Patients with prior atypical endometrial hyperplasia demonstrated significantly higher expression of proliferative (cyclin D1, CDK4, and P16) and immune-suppressive markers (FOXP3 and CD163) and a 66% progression to carcinoma. Clinical background factors were statistically adjusted and did not alter the overall progression trend.
Conclusion: The stepwise evolution from benign endometrial lesions to carcinoma is driven by coordinated proliferative and immune microenvironmental shifts, potentiated by a history of atypical endometrial hyperplasia. Integrating immunohistochemical and clinical risk factors may enhance early identification and surveillance of patients at high risk for endometriosis-associated carcinoma.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








