Clinicopathologic and Immunohistochemical Correlates of Disease-Free Survival in Endometrial Stromal Sarcomas: A Multicenter Retrospective Study From 2017 to 2025
DOI:
https://doi.org/10.14740/jocmr6360Keywords:
Endometrial stromal sarcoma, CD163, Ki67, CyclinD1, Disease-free survival, Immunohistochemistry, Prognostic biomarkers, Multicenter cohortAbstract
Background: Endometrial stromal tumors (ESTs) represent a heterogeneous group of uterine mesenchymal neoplasms with variable clinical outcomes. Although histological grading is a cornerstone for prognosis, the contribution of proliferative and immune microenvironment markers remains incompletely defined.
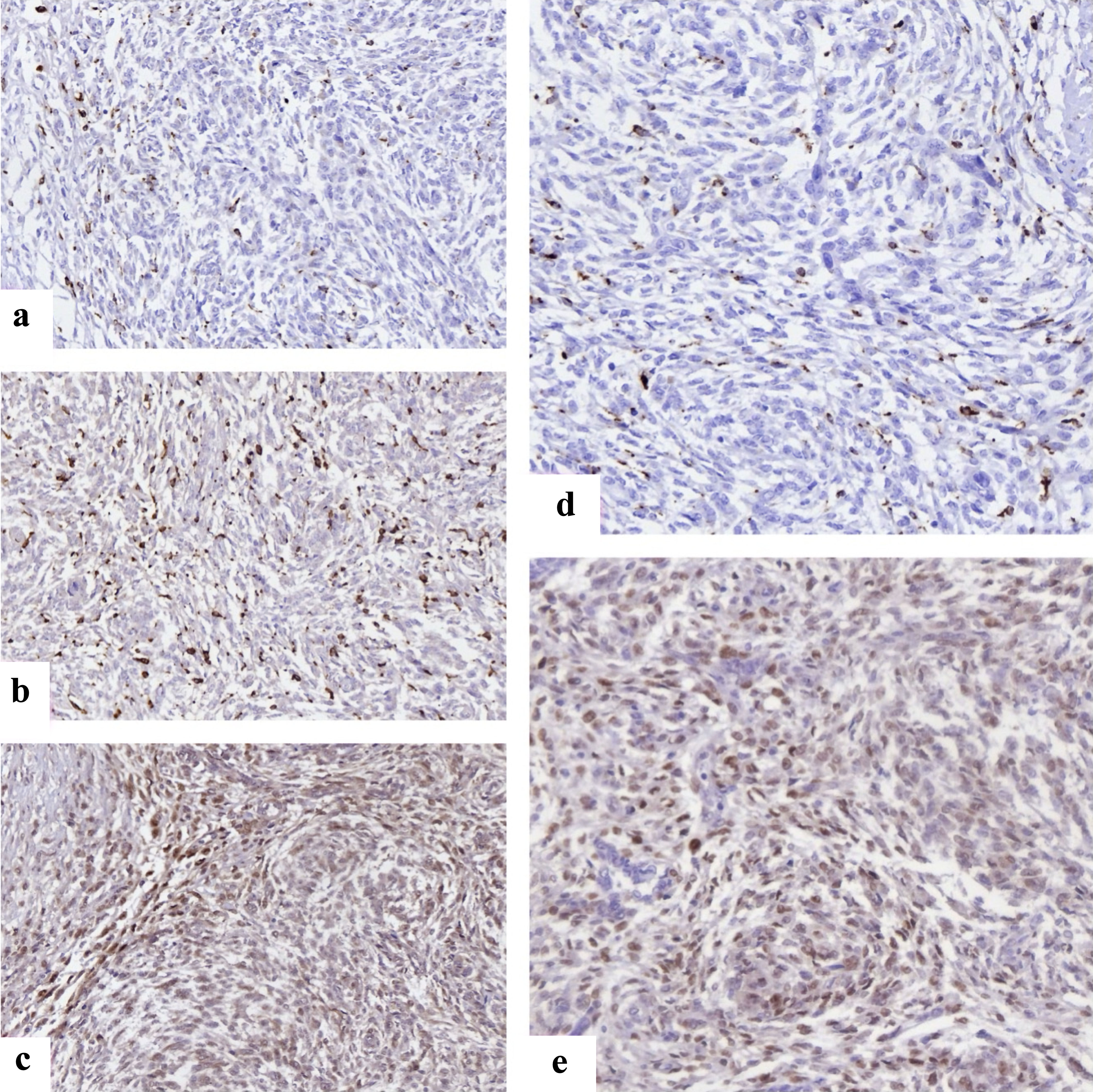
Methods: We retrospectively analyzed 90 patients diagnosed with endometrial stromal nodule (ESN) (n = 30), low-grade endometrial stromal sarcoma (LG-ESS, n = 30), and high-grade endometrial stromal sarcoma (HG-ESS, n = 30) between 2017 and 2025 across 35 public and private clinics in four Georgian cities. All specimens underwent standardized immunohistochemistry for estrogen receptor (ER), progesterone receptor (PR), Ki67, cyclinD1, cyclin-dependent kinase 4 (CDK4), CD117, forkhead box P3 (FOXP3), CD163, and CD34. Disease-free survival (DFS) was calculated from date of surgery to recurrence/metastasis. Kaplan-Meier curves and log-rank tests were used to assess survival differences, and data-driven cutoffs (Youden index) were employed to stratify biomarker expression. Multivariable Cox proportional hazards regression was applied to identify independent predictors of recurrence.
Results: Median follow-up was 55 months. DFS significantly differed by histology: not reached for ESN, 20.0 months for LG-ESS, and 5.0 months for HG-ESS (log-rank P < 0.0001). High Ki67, cyclinD1, CDK4, CD117, FOXP3, and CD163 predicted shortened DFS, while ER/PR expression correlated with prolonged DFS (all P < 0.0001). In adjusted models, lymphovascular space invasion (LVSI) (odds ratio (OR): 3.59, 95% confidence interval (CI): 3.21 - 3.87), Ki67 (OR: 4.65, 4.08 - 5.10), tumor necrosis (OR: 2.39, 2.06 - 2.79), cyclinD1 (OR: 2.20, 1.99 - 2.43), and CD163 (OR: 2.06, 1.72 - 2.51) remained independently associated with recurrence.
Conclusions: Beyond histological grade, proliferative signaling and M2 macrophage polarization strongly influence recurrence risk in ESS. These findings highlight potential diagnostic and therapeutic targets, suggesting integration of immune and cell-cycle biomarkers into future risk stratification models.
Published
Issue
Section
License
Copyright (c) 2026 The authors

This work is licensed under a Creative Commons Attribution 4.0 International License.








